Interfacially Cross-Linked Polydopamine/Polybenzimidazole Composite Membranes for Organic Solvent Nanofiltration
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
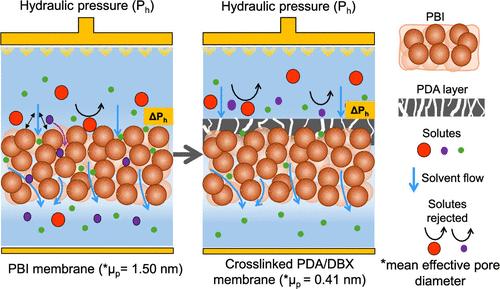
Interfacial cross-linking was used to prepare composite organic solvent nanofiltration (OSN) membranes comprising a polydopamine (PDA) active layer formed on a polybenzimidazole (PBI) substrate. Dibromo-p-xylene (DBX) was employed as a cross-linking agent to make the composite membranes chemically stable against harsh polar aprotic solvents. The interfacial cross-linking of PDA/PBI allowed for finely tuning the molecular weight cutoff (MWCO) of the membrane, resulting in a membrane with precise molecular separation capabilities for OSN. The morphology and surface properties of the membranes were characterized, and a membrane with a MWCO of 286 Da was investigated for OSN of a series of solvents. The membrane permeance was in the order of acetonitrile (MeCN) > methanol (MeOH) > acetone > toluene > dimethylformamide (DMF) > heptane > ethanol (EtOH) > isopropanol (IPA) > tetrahydrofuran (THF). The membranes displayed a sharp pore size distribution, yielding a rejection rate of over 99% for Rose Bengal (RB, MW 1020 g/mol) and Remazol brilliant blue (RBB, MW 626.5 g/mol) from DMF and EtOH solutions. When it came to methyl orange (MO, MW 327.3 g/mol) that had a molecular weight closer to the MWCO of the membrane, the membrane still displayed a high rejection rate of 95% and 99% in nanofiltrating solvents DMF and EtOH, respectively. In addition, it was demonstrated that the membrane was able to effectively fractionate mixed solutes having molecular weights appropriate for the MWCO rating of the membrane during OSN.
用于有机溶剂纳滤的界面交联聚多巴胺/聚苯并咪唑复合膜
利用界面交联法制备了复合有机溶剂纳滤膜(OSN),该膜由聚多巴胺(PDA)活性层和聚苯并咪唑(PBI)基底构成。采用二溴对二甲苯(DBX)作为交联剂,使复合膜对苛刻的极性烷基溶剂具有化学稳定性。PDA/PBI 的界面交联允许微调膜的分子量截止值 (MWCO),从而使膜具有精确的分子分离能力,可用于 OSN。对膜的形态和表面特性进行了表征,并研究了一种截留分子量为 286 Da 的膜对一系列溶剂的 OSN 作用。膜的渗透性依次为乙腈(MeCN);甲醇(MeOH);丙酮;甲苯;二甲基甲酰胺(DMF);庚烷;乙醇(EtOH);异丙醇(IPA);四氢呋喃(THF)。膜显示出清晰的孔径分布,对 DMF 和 EtOH 溶液中的玫瑰红(RB,截留分子量为 1020 克/摩尔)和雷马唑艳蓝(RBB,截留分子量为 626.5 克/摩尔)的截留率超过 99%。对于分子量更接近膜截留分子量的甲基橙(MO,截留分子量为 327.3 g/mol),该膜在纳滤溶剂 DMF 和 EtOH 中的截留率仍然很高,分别为 95% 和 99%。此外,实验还证明,在 OSN 过程中,膜能够有效地分馏分子量适合膜截留分子量等级的混合溶质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


