Structure–Activity Relationship Studies of Imidazo[1′,2′:1,6]pyrido[2,3-d]pyrimidine Derivatives to Develop Selective FGFR Inhibitors as Anticancer Agents for FGF19-overexpressed Hepatocellular Carcinoma
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
The aberrant activation of fibroblast growth factor (FGF) and FGF receptor (FGFR)-mediated signaling pathways are associated with cancer development, including hepatocellular carcinoma (HCC). A novel series of imidazo[1′,2′:1,6]pyrido[2,3-d]pyrimidine, containing an acrylamide covalent warhead, were synthesized as selective FGFR 1-4 inhibitors. Compound 7n was identified as the most potent inhibitor against FGFR1, 2, and 4, with IC50 values of 8/4 nM (FGFR1/2) and 3.8 nM (FGFR4), and the covalent docking analyses suggested that 7n form a covalent adduct with cysteine residue on the hinge or p-loop of FGFR. Compound 7n exhibited a favorable pharmacokinetic profile and significant in vivo antitumor efficacy in human liver cancer xenograft mouse models (xenograft, FGF/FGFR-dependent HCC cells).
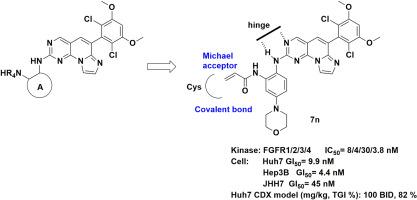
咪唑并[1′,2′:1,6]吡啶并[2,3-d]嘧啶衍生物的结构-活性关系研究,开发作为 FGF19 表达过高的肝细胞癌抗癌药物的选择性 FGFR 抑制剂
成纤维细胞生长因子(FGF)和成纤维细胞生长因子受体(FGFR)介导的信号通路的异常激活与包括肝细胞癌(HCC)在内的癌症发展有关。研究人员合成了一系列含有丙烯酰胺共价弹头的新型咪唑并[1′,2′:1,6]吡啶并[2,3-d]嘧啶,作为选择性 FGFR 1-4 抑制剂。化合物 7n 被鉴定为对 FGFR1、2 和 4 最有效的抑制剂,其 IC50 值分别为 8/4 nM(FGFR1/2)和 3.8 nM(FGFR4),共价对接分析表明 7n 与 FGFR 铰链或 p 环上的半胱氨酸残基形成共价加合物。化合物 7n 在人肝癌异种移植小鼠模型(异种移植,FGF/FGFR 依赖性 HCC 细胞)中表现出良好的药代动力学特征和显著的体内抗肿瘤疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


