Enantiomeric C-6 fluorinated swainsonine derivatives as highly selective and potent inhibitors of α-mannosidase and α-l-rhamnosidase: Design, synthesis and structure-activity relationship study
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Six C-6 fluorinated d-swainsonine derivatives and their enantiomers have been designed based on initial docking calculations, and synthesized from enantiomeric ribose-derived aldehydes, respectively. Glycosidase inhibition assay of these derivatives with d-swainsonine (1) and l-swainsonine (ent-1) as contrasts found that the C-6 fluorinated d-swainsonine derivatives with C-8 configurations as R (α) showed specific and potent inhibitions of jack bean α-mannosidase (model enzyme of Golgi α-mannosidase II); whereas their enantiomers with C-8 configurations as S (β) were powerful and selective α-l-rhamnosidase inhibitors. Molecular docking calculations found the C-6 fluorinatedd-swainsonine derivatives 21, 24 and 25 with highly coincident binding conformations with d-swainsonine (1) in their interactions with the active site of α-mannosidase (PDB ID: 1HWW). Reliability of the docking results were confirmed by Molecular Dynamics (MD) simulation. Additionally, solid interactions with residues Gln-392 and Tyr-393 in the active site of α-l-rhamnosidase (PDB ID: 3W5N) were proved to be vital for potent α-l-rhamnosidase inhibitions of the l-swainsonine derivatives. The role of C-6 fluorines in swainsonine derivatives well demonstrated the “mimic effect” of fluorine to hydrogen by minimal influence on the binding conformations and effective compensation for any possible lost interactions. This work contributes to a comprehensive understanding of the structure-activity relationship (SAR) of the fluorinated swainsonines and ever reported branched swainsonines, and has laid good foundation for development of more potent α-mannosidase and α-l-rhamnosidase inhibitors.
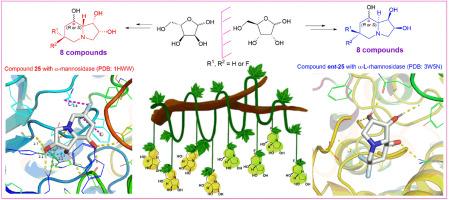
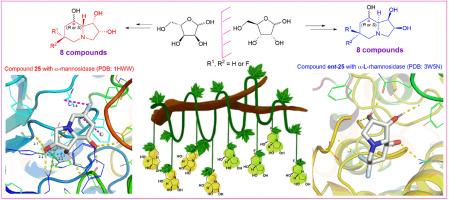
作为α-甘露糖苷酶和α-l-鼠李糖苷酶高选择性强效抑制剂的对映体C-6氟化莽草酸衍生物:设计、合成和结构-活性关系研究
根据初步的对接计算,我们设计了六种 C-6 氟化 d-瑞香素衍生物及其对映体,并分别从对映体核糖衍生醛中合成。以 d-岩苏宁(1)和 l-岩苏宁(ent-1)作为对比,对这些衍生物进行糖苷酶抑制实验,发现 C-6 氟化 d-岩苏宁衍生物以 C-8 构型为 R (α) 对胡豆 α-甘露糖苷酶(高尔基体 α-甘露糖苷酶 II 的模型酶)具有特异性和强效的抑制作用;而其 C-8 构型为 S (β) 的对映体则是强效且具有选择性的 α-l-rhamnosidase 抑制剂。分子对接计算发现,在与α-甘露糖苷酶(PDB ID:1HWW)活性位点的相互作用中,C-6氟化d-岩白菜素衍生物21、24和25与d-岩白菜素(1)的结合构象高度重合。分子动力学(MD)模拟证实了对接结果的可靠性。此外,与 α-鼠李糖苷酶(PDB ID:3W5N)活性位点残基 Gln-392 和 Tyr-393 的牢固相互作用被证明是 l-岩白菜素衍生物有效抑制 α-鼠李糖苷酶的关键。C-6 氟在娃素宁衍生物中的作用充分证明了氟对氢的 "模仿效应",它对结合构象的影响极小,并能有效补偿任何可能失去的相互作用。这项研究工作有助于全面了解氟化獐牙菜宁类和曾报道过的支链獐牙菜宁类的结构-活性关系(SAR),并为开发更有效的 α-甘露糖苷酶和 α-l-rhamnosidase 抑制剂奠定了良好的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


