Structural insights into tryptamine psychedelics: The role of hydroxyl indole ring site in 5-HT2A receptor activation and psychedelic-like activity
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Recent advancements in the study of mushroom-derived tryptamines, particularly psilocybin and its metabolite psilocin, highlight their unique psychedelic properties and potential therapeutic applications, especially for mental health conditions like depression. This study examines how the position of the hydroxyl group on the indole ring affects the 5-HT2A receptor activity and psychedelic-like effects of psilocin analogs. Chemically synthesized psilocin (1) and its analogs bufotenine (2), 6-OH-DMT (3), and 7-OH-DMT (4) were assessed for 5-HT2A receptor agonistic activity using the Gαq-Gγ dissociation bioluminescence resonance energy transfer (BRET) assay and for psychedelic-like effects through the head-twitch response assay. Results show that compounds with hydroxyl group at the 4th and 5th positions exhibit significantly higher 5-HT2A agonistic and psychedelic-like activities than those with hydroxyl group at the 6th and 7th positions. Funnel metadynamics simulations revealed that psilocin (1) and bufotenine (2) have lower binding free energies, correlating with experimental data. Analysis of the simulation trajectories reveals that the formation of a hydrogen bond with residue L229 is crucial for guiding psilocin (1) and bufotenine (2) into the 5-HT2AR binding site. In contrast, analogs 3 and 4, which lack this interaction, fail to be directed into the orthosteric site. Furthermore, psilocin (1) and bufotenine (2) establish a stable salt bridge and hydrogen bond with residue D155. These interactions are more stable compared to those formed by ligands 3 and 4, contributing to the latter's poor 5-HT2AR activities. These findings underscore the critical role of the hydroxyl group position on the indole ring in modulating 5-HT2A receptor activity and the corresponding psychedelic-like effects, offering valuable insights for the development of targeted therapeutics.
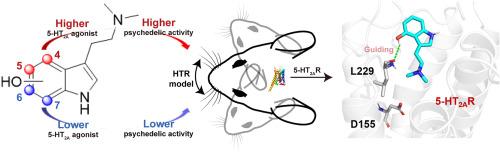
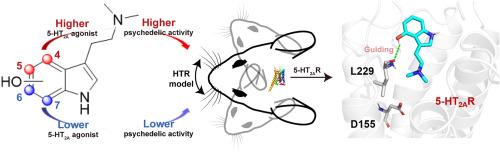
色胺迷幻剂的结构洞察:羟基吲哚环位点在 5-HT2A 受体激活和迷幻样活性中的作用
最近,对蘑菇衍生色胺,特别是西洛赛宾及其代谢物西洛辛的研究取得了进展,突显了它们独特的迷幻特性和潜在的治疗应用,尤其是对抑郁症等精神疾病的治疗。本研究探讨了吲哚环上羟基的位置如何影响 5-HT2A 受体的活性以及迷幻药类似物的迷幻效果。研究人员使用 Gαq-Gγ 解离生物发光共振能量转移(BRET)测定法评估了化学合成的西洛辛(1)及其类似物布福噻宁(2)、6-OH-DMT(3)和 7-OH-DMT(4)的 5-HT2A 受体激动活性,并通过头部抽搐反应测定法评估了它们的迷幻样效应。结果表明,羟基位于第 4 和第 5 位的化合物的 5-HT2A 激动活性和类迷幻活性明显高于羟基位于第 6 和第 7 位的化合物。漏斗元动力学模拟显示,西洛辛(1)和布福替宁(2)的结合自由能较低,这与实验数据相关。对模拟轨迹的分析表明,与残基 L229 形成氢键是引导 psilocin(1)和 bufotenine(2)进入 5-HT2AR 结合位点的关键。相比之下,缺乏这种相互作用的类似物 3 和 4 则无法被引导到正交位点。此外,西洛辛(1)和布福滕宁(2)还与残基 D155 建立了稳定的盐桥和氢键。这些相互作用比配体 3 和 4 形成的相互作用更稳定,从而导致后者的 5-HT2AR 活性较差。这些发现强调了吲哚环上羟基位置在调节 5-HT2A 受体活性和相应的迷幻样效应中的关键作用,为开发靶向治疗药物提供了宝贵的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


