Synergistic interplay of CuOx and Pd nanodots on TiO2 for efficient and highly selective photocatalytic oxidation of CH4 to oxygenates with O2
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
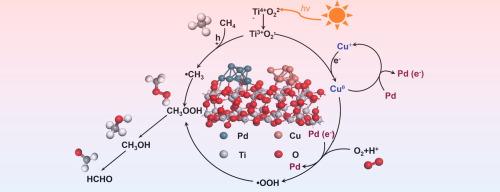
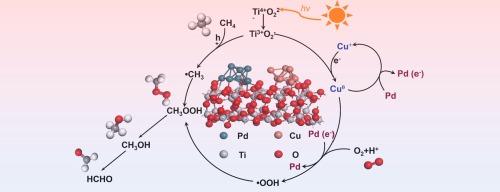
Direct photocatalytic methane oxidation to produce liquid oxygenates offers a promising approach for the upgrading of abundant methane under mild conditions, yet it remains a formidable challenge in achieving high reaction rates while maintaining high selectivity. Herein, we report the highly dispersed CuOx and Pd nanodots decorated TiO2 for photocatalytic oxidation of CH4 with O2 at room temperature, which exhibits a remarkable C1 oxygenates production rate of 39.5 mmol·g−1·h−1 with a nearly 100 % selectivity, outperforming most of the state-of-the-art photocatalysts. Both experimental and theoretical studies suggest that the impressive photocatalytic performance is attributed to the synergy of Cu+ species and Pd nanodots. Cu+ species not only promote the interfacial electrons transfer from TiO2 to Pd, but also mediate CH4 oxidation reaction to avoid overoxidation of oxygenates to CO2, while the resulting electron-rich Pd sites boost the production of primary products (CH3OOH and CH3OH) by lowering the reaction energy. This work provides a new pathway for developing highly efficient photocatalysts for the selective conversion of methane to value-added chemicals by designing bimetallic cocatalysts.
铜氧化物和钯纳米点在二氧化钛上的协同作用可高效、高选择性地光催化氧化 CH4 与 O2 生成含氧化合物
直接光催化甲烷氧化制取液态含氧化合物为在温和条件下提纯丰富的甲烷提供了一种前景广阔的方法,但要在保持高选择性的同时实现高反应速率仍是一项艰巨的挑战。在此,我们报告了高度分散的 CuOx 和 Pd 纳米点装饰的 TiO2 在室温下用 O2 光催化氧化 CH4 的情况,其 C1 含氧化合物的生产率高达 39.5 mmol-g-1-h-1,选择性接近 100%,优于大多数最先进的光催化剂。实验和理论研究都表明,令人印象深刻的光催化性能归功于 Cu+ 物种和钯纳米点的协同作用。Cu+ 物种不仅能促进界面电子从 TiO2 转移到 Pd,还能介导 CH4 氧化反应,避免含氧化合物过度氧化成 CO2,而由此产生的富电子 Pd 位点则能通过降低反应能量促进初级产品(CH3OOH 和 CH3OH)的生成。这项工作为通过设计双金属协同催化剂来开发高效光催化剂提供了一条新途径,可用于将甲烷选择性转化为高附加值化学品。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


