Unusually high selectivity (100 %) of photocatalytic CC coupling achieved by instant reverse reduction of byproducts
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
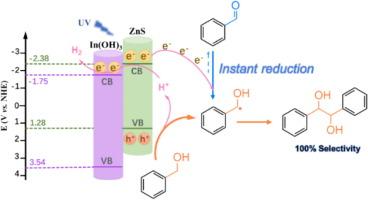
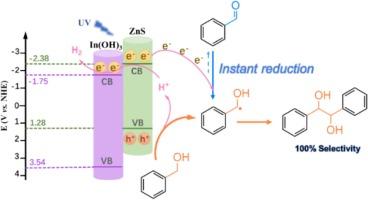
The photocatalytic C![]() C coupling of benzyl alcohol (BA) into hydrobenzoin (HB), is appealing to obtain high-value chemicals. However, the selectivity of HB is still low due to the inevitable formation of benzaldehyde. Herein, we report In(OH)3-ZnS photocatalyst for C
C coupling of benzyl alcohol (BA) into hydrobenzoin (HB), is appealing to obtain high-value chemicals. However, the selectivity of HB is still low due to the inevitable formation of benzaldehyde. Herein, we report In(OH)3-ZnS photocatalyst for C![]() C coupling of BA into HB with very high selectivity (∼100 %). The introduction of In(OH)3 onto ZnS with stable interaction facilitates light harvesting and separation of photo-excited charges. As a result, BA conversion on optimized In(0.1)-ZnS catalyst (73 %) is much higher than ZnS (29 %). Besides, the surface hydroxyl groups derived from In(OH)3 enables the facile desorption of
C coupling of BA into HB with very high selectivity (∼100 %). The introduction of In(OH)3 onto ZnS with stable interaction facilitates light harvesting and separation of photo-excited charges. As a result, BA conversion on optimized In(0.1)-ZnS catalyst (73 %) is much higher than ZnS (29 %). Besides, the surface hydroxyl groups derived from In(OH)3 enables the facile desorption of ![]() CH(OH)Ph radical. Therefore, the over oxidation of
CH(OH)Ph radical. Therefore, the over oxidation of ![]() CH(OH)Ph radical into by-product of benzaldehyde can be effectively inhibited. More significantly, in-situ FTIR spectra and reduction of by-product manifest the instant reverse reduction process of benzaldehyde into
CH(OH)Ph radical into by-product of benzaldehyde can be effectively inhibited. More significantly, in-situ FTIR spectra and reduction of by-product manifest the instant reverse reduction process of benzaldehyde into ![]() CH(OH)Ph radical during C
CH(OH)Ph radical during C![]() C coupling of BA, which is the key to realizing satisfied HB selectivity (100 %). Theoretical simulations reveal that the weak adsorption of
C coupling of BA, which is the key to realizing satisfied HB selectivity (100 %). Theoretical simulations reveal that the weak adsorption of ![]() CH(OH)Ph radical over catalyst and the high energy barrier of over-oxidation of
CH(OH)Ph radical over catalyst and the high energy barrier of over-oxidation of ![]() CH(OH)Ph into benzaldehyde contributes to the formation of highly selective coupling products. This work will inspire new insights to design rational photoredox systems for organic transformations with high selectivity.
CH(OH)Ph into benzaldehyde contributes to the formation of highly selective coupling products. This work will inspire new insights to design rational photoredox systems for organic transformations with high selectivity.
通过瞬间反向还原副产物,实现光催化 CC 偶联的超高选择性(100
通过光催化 CC 将苯甲醇(BA)偶联成氢安息香(HB),对获得高价值化学品具有吸引力。然而,由于不可避免地会形成苯甲醛,因此 HB 的选择性仍然很低。在此,我们报告了 In(OH)3-ZnS 光催化剂用于将 BA 以极高的选择性(∼100 %)耦合到 HB 中。在 ZnS 上引入 In(OH)3 具有稳定的相互作用,有利于光收集和光激发电荷的分离。因此,优化 In(0.1)-ZnS 催化剂上的 BA 转化率(73%)远高于 ZnS(29%)。此外,In(OH)3 产生的表面羟基使 CH(OH)Ph 自由基易于解吸。因此,CH(OH)Ph 自由基过度氧化成苯甲醛副产物的过程可以得到有效抑制。更重要的是,原位傅立叶变换红外光谱和副产物的还原表明,在 BA 的 CC 偶联过程中,苯甲醛瞬间反向还原为 CH(OH)Ph 自由基,这是实现满意的 HB 选择性(100%)的关键。理论模拟揭示了催化剂对 CH(OH)Ph 自由基的弱吸附性和 CH(OH)Ph 过度氧化成苯甲醛的高能垒有助于形成高选择性的偶联产物。这项工作将为设计用于高选择性有机转化的合理光氧化系统提供新的启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


