Metal cation exchange with zeolitic acid sites modulates hydrocarbon pool propagation during CO2 hydrogenation
IF 11.6
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
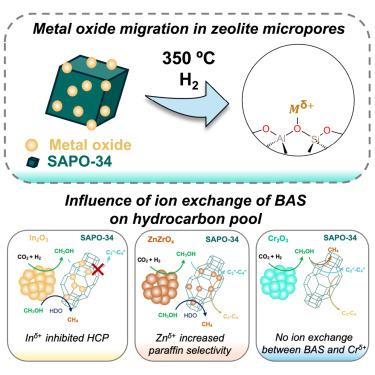
We demonstrate that the exchange of zeolitic Brønsted acid sites (BASs) with cations from metal oxides plays a pivotal role in the propagation of hydrocarbon pools (HCPs) during CO2 hydrogenation. We probed the likelihood of In2O3, ZnZrOx, and Cr2O3 migration and their cation exchange with BASs of a silicoaluminophosphate, SAPO-34, by integrating them at nanoscale proximity (∼1,400 nm). Analysis with NH3 temperature-programmed desorption and transmission Fourier transform infrared spectroscopy showed ion exchange of BASs with Inδ+ and Znδ+ but not for Crδ+. We measured the C3/C2 hydrocarbon ratio (indicating relative propagation of olefin to aromatic cycles) and paraffin-to-olefin ratio, which revealed that Inδ+ species inhibited HCPs inside the channels of SAPO-34, while Znδ+ species enhanced hydrogen transfer and secondary hydrogenation. Combining reactivity data with occluded hydrocarbon analysis and 13C solid-state nuclear magnetic resonance spectroscopy, we show that ion-exchanged species affect HCP propagation. Overall, our work provides insights for the rational integration of bifunctional catalysts.
金属阳离子与沸石酸位点的交换调节二氧化碳加氢过程中碳氢化合物池的扩散
我们证明,在二氧化碳氢化过程中,沸石布氏酸位点(BAS)与金属氧化物阳离子的交换在碳氢化合物池(HCP)的传播中起着关键作用。我们通过将 In2O3、ZnZrOx 和 Cr2O3 与硅铝磷酸盐 SAPO-34 的 BASs 进行纳米级(∼1,400 nm)整合,探究了它们迁移的可能性及其与 BASs 的阳离子交换。利用 NH3 温度编程解吸和透射傅立叶变换红外光谱进行的分析表明,BAS 与 Inδ+ 和 Znδ+ 发生了离子交换,但与 Crδ+ 没有发生离子交换。我们测量了 C3/C2 碳氢化合物比率(表明烯烃到芳烃循环的相对传播)和石蜡与烯烃比率,结果显示 Inδ+ 物种抑制了 SAPO-34 通道内的 HCP,而 Znδ+ 物种增强了氢转移和二次氢化。结合反应性数据、闭塞碳氢化合物分析和 13C 固态核磁共振光谱,我们发现离子交换物种会影响 HCP 的传播。总之,我们的工作为合理整合双功能催化剂提供了启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


