Functionalized TEVS-EGDMA microspheres for efficient cadmium(II) removal: Synthesis, characterization, and adsorption performance
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The development of new functionalized adsorbents in the form of polymeric microspheres, based on triethoxyvinylsilane (TEVS) and ethylene glycol dimethacrylate (EGDMA), has been achieved. These adsorbents were created by incorporating coumarin additives, synthesized in an environmentally friendly manner, into the TEVS-EGDMA polymer matrix. This includes coumarin-3-carboxylic acid and its ester derivatives (CRM1-4). The newly developed adsorbents (TEVS-EGDMA-CRM1, TEVS-EGDMA-CRM2, TEVS-EGDMA-CRM3, and TEVS-EGDMA-CRM4) have been applied for the removal of Cd(II) from aqueous solutions. The material was characterized using spectral techniques like pHzpc, ATR-FTIR, SEM with EDS, DSC, and specific surface area (SBET). The maximum adsorption capacity for Cd(II) is 58.73, 59.03, 59.16, 63.07 and 64.26 mg/g at pH 6.5, pHzpc 6.16, 6.28, 6.40, 6.49, and 6.63, and equilibration time of 240 min, respectively. The addition of coumarins to the microspheres improves the thermal resistance of samples. The kinetics are well-fitted by the pseudo-second-order kinetic model (R2 > 0.99). The equilibrium adsorption data are fitted by the Langmuir, Freundlich and Dubinin-Raduskevich isotherms. The adsorption capacities calculated from the Langmuir model agree with the experimental results.
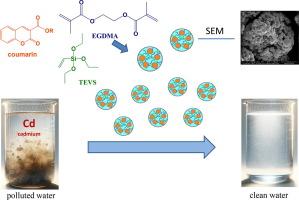
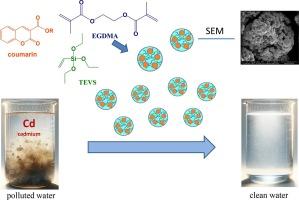
用于高效去除镉(II)的功能化 TEVS-EGDMA 微球:合成、表征和吸附性能
以三乙氧基乙烯基硅烷(TEVS)和乙二醇二甲基丙烯酸酯(EGDMA)为基础,开发出了聚合物微球形式的新型功能化吸附剂。这些吸附剂是通过在 TEVS-EGDMA 聚合物基质中加入以环保方式合成的香豆素添加剂而制成的。其中包括香豆素-3-羧酸及其酯类衍生物(CRM1-4)。新开发的吸附剂(TEVS-EGDMA-CRM1、TEVS-EGDMA-CRM2、TEVS-EGDMA-CRM3 和 TEVS-EGDMA-CRM4)已被用于去除水溶液中的镉(II)。使用 pHzpc、ATR-FTIR、SEM(带 EDS)、DSC 和比表面积 (SBET) 等光谱技术对材料进行了表征。在 pH 值为 6.5,pHzpc 为 6.16、6.28、6.40、6.49 和 6.63,平衡时间为 240 分钟时,镉(II)的最大吸附容量分别为 58.73、59.03、59.16、63.07 和 64.26 毫克/克。在微球中添加香豆素可提高样品的耐热性。假二阶动力学模型(R2 >0.99)很好地拟合了动力学。平衡吸附数据由 Langmuir、Freundlich 和 Dubinin-Raduskevich 等温线拟合。根据 Langmuir 模型计算出的吸附容量与实验结果一致。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


