High areal capacity and long-life Sn anode enabled by tuning electrolyte solvation chemistry and interfacial adsorbed molecular layer
IF 18.9
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
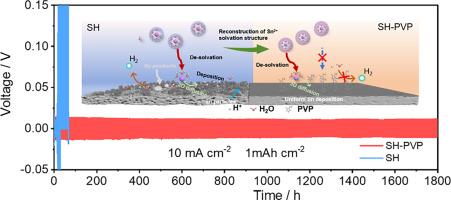
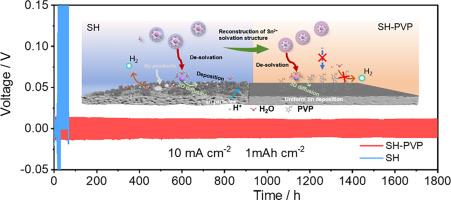
Tin (Sn) is an appealing metal anode for aqueous batteries (ABs) due to its high theoretical capacity, elevated hydrogen overpotential, affordability and environmentally friendly nature. However, the parasitic reaction and dead Sn formation are two critical issues that impede the practical application of Sn metal batteries. Herein, we demonstrate that the addition of trace amount of polyvinylpyrrolidone (PVP, 1 mM) into the pristine electrolyte can effectively solve these issues. Specifically, the PVP additive can reshape the structure of Sn2+ solvation sheath to accelerate cations migration and suppress water-induced side reaction and the formation of hydroxide sulfate. Additionally, the preferential adsorption of PVP at the interface also promotes the three-dimensional (3D) diffusion of Sn2+, facilitating uniform Sn deposition. As a result, symmetric cells with PVP additive in the electrolyte deliver stable cycling for up to 1800 h at 10 mA cm−2/1 mAh cm−2 or 230 h at 5 mA cm−2/10 mAh cm−2. The designed electrolyte also enables the MnO2//Sn full battery to maintain a discharge capacity of 0.92 mAh cm−2 over 3000 cycles at current density of 6 mA cm−2 and supports the stable cycling of PbO2//Sn full battery for 230 cycles under the high capacity of 10 mAh cm−2.
通过调节电解质溶解化学和界面吸附分子层实现高面积容量和长寿命锡阳极
锡(Sn)具有理论容量高、氢过电位高、价格低廉和环保等优点,是水电池(ABs)的理想金属阳极。然而,寄生反应和死锡的形成是阻碍锡金属电池实际应用的两个关键问题。在此,我们证明了在原始电解液中添加微量聚乙烯吡咯烷酮(PVP,1 mM)可有效解决这些问题。具体来说,聚乙烯吡咯烷酮添加剂可以重塑 Sn2+ 溶解鞘的结构,加速阳离子迁移,抑制水引起的副反应和硫酸氢氧根的形成。此外,PVP 在界面上的优先吸附作用还能促进 Sn2+ 的三维(3D)扩散,从而促进 Sn 的均匀沉积。因此,在电解液中添加 PVP 的对称电池在 10 mA cm-2/1 mAh cm-2 的条件下可稳定循环 1800 小时,在 5 mA cm-2/10 mAh cm-2 的条件下可稳定循环 230 小时。所设计的电解液还能使 MnO2//Sn 全电池在电流密度为 6 mA cm-2 的条件下,在 3000 次循环中保持 0.92 mAh cm-2 的放电容量,并支持 PbO2//Sn 全电池在 10 mAh cm-2 的高容量条件下稳定循环 230 次。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


