Iron-catalysed intramolecular C(sp3)–H amination of alkyl azides
IF 4.3
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Iron-catalysed intramolecular C(sp3)–H amination of alkyl azides (N3R, R = alkyl) via the iron–alkylnitrene/alkylimido (Fe(NR)) intermediate, is an appealing synthetic approach for the synthesis of various N-heterocycles. This approach provides a direct atom-economy strategy for constructing C(sp3)–N bonds, with nitrogen gas as the only by-product and iron is a biocompatible, cheap, and earth-abundant metal. However, C(sp3)–H amination with alkyl azides is challenging because alkyl nitrenes readily undergo 1,2-hydride migration to imines. This article summarizes recent major advances in this field in terms of catalyst design, substrate scope expansion, stereoselectivity control, understanding of key reaction intermediates, and applications in the synthesis of complex natural products and pharmaceuticals.
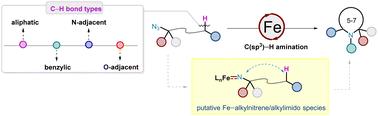

铁催化的烷基叠氮化物分子内 C(sp3)-H 氨基化反应
通过铁-烷基硝基/烷基亚胺(Fe(NR))中间体,铁催化烷基叠氮化物(N3R,R = 烷基)的分子内 C(sp3)-H-胺化反应,是合成各种 N-杂环的一种极具吸引力的合成方法。这种方法为构建 C(sp3)-N 键提供了一种直接的原子经济策略,氮气是唯一的副产品,而铁是一种生物相容性好、廉价且地球资源丰富的金属。然而,C(sp3)-H 与烷基叠氮化物的胺化具有挑战性,因为烷基腈很容易发生 1,2-酸酐向亚胺的迁移。本文从催化剂设计、底物范围扩展、立体选择性控制、对关键反应中间体的理解以及在复杂天然产物和药物合成中的应用等方面总结了该领域的最新主要进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


