Lone Pair−π Interactions in Organic Reactions
IF 51.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Noncovalent interactions between a lone pair of electrons and π systems can be categorized into two types based on the nature of π systems. Lone pair−π(C═O) interactions with π systems of unsaturated, polarized bonds are primarily attributed to orbital interactions, whereas lone pair−π(Ar) interactions with π systems of aromatic functional groups result from electrostatic attractions (for electron-deficient aryls) or dispersion attractions and Pauli repulsions (for electron-rich/neutral aryls). Unlike well-established noncovalent interactions, lone pair−π interactions have been comparatively underappreciated or less used to influence reaction outcomes. This review emphasizes experimental and computational studies aimed at integrating lone pair−π interactions into the design of catalytic systems and utilizing these interactions to regulate the reactivity and selectivity of chemical transformations. The role of lone pair−π interactions is highlighted in the stabilization or destabilization of transition states and ground-state binding. Examples influenced by lone pair−π interactions with both unsaturated, polarized bonds and aromatic rings as π systems are included. At variance with previous reviews, the present review is not structured according to the physical origin of particular classes of lone pair−π interactions but is divided into chapters according to ways in which lone pair−π interactions affect kinetics and/or selectivity of reactions.
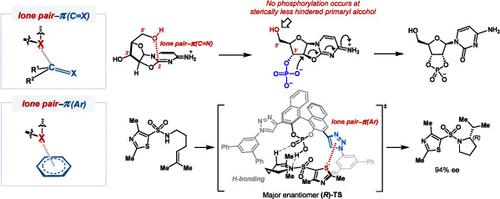
有机反应中的孤对π相互作用
孤对电子与 π 系统之间的非共价相互作用可根据 π 系统的性质分为两类。孤对电子-π(C═O)与不饱和、极化键的π系统之间的相互作用主要归因于轨道相互作用,而孤对电子-π(Ar)与芳香官能团的π系统之间的相互作用则来自静电吸引(对于电子缺乏的芳基)或色散吸引和保利排斥(对于电子丰富/中性的芳基)。与公认的非共价相互作用不同,孤对-π相互作用一直未得到足够重视,或较少用于影响反应结果。本综述强调实验和计算研究,旨在将孤对π相互作用整合到催化系统的设计中,并利用这些相互作用来调节化学转化的反应性和选择性。研究强调了孤对π相互作用在稳定或破坏过渡态和基态结合方面的作用。其中包括受孤对π相互作用影响的不饱和、极化键和作为π系统的芳香环的例子。与以往的综述不同的是,本综述不是根据孤对-π相互作用特定类别的物理起源来编排的,而是根据孤对-π相互作用影响反应动力学和/或选择性的方式来划分章节的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Reviews
化学-化学综合
CiteScore
106.00
自引率
1.10%
发文量
278
审稿时长
4.3 months
期刊介绍:
Chemical Reviews is a highly regarded and highest-ranked journal covering the general topic of chemistry. Its mission is to provide comprehensive, authoritative, critical, and readable reviews of important recent research in organic, inorganic, physical, analytical, theoretical, and biological chemistry.
Since 1985, Chemical Reviews has also published periodic thematic issues that focus on a single theme or direction of emerging research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


