Modulating Coordination Environment of Cobalt-Based Spinel Octahedral Metal Sites to Boost Metal–Oxygen Bond Covalency for Reversible Lithium–Oxygen Batteries
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
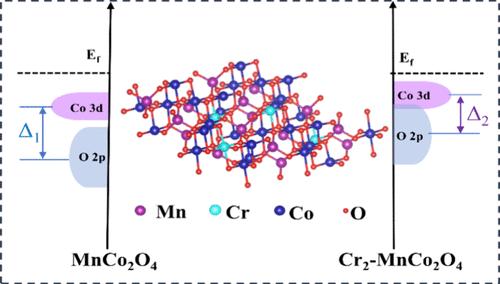
The intrinsic catalytic activity of conventional spinel electrocatalysts hinders their electrocatalytic outcomes in lithium–oxygen batteries (LOBs), despite their appeal due to compositional variety and structural adaptability. In this work, we reveal that the electrocatalytic activities of these catalysts can be inherently enhanced by modulating metal–oxygen (M–O) bond covalency interactions through the introduction of the Cr element into the MnCo2O4 octahedral sites (MnCr0.5Co1.5O4). The introduction of Cr3+ directly alters the coordination structure of Co octahedral sites, which increases the Co3+–O distance and reduces the lattice symmetry, resulting in enhanced covalency interactions of the M–O bond. Computational analysis supports the effectiveness of Cr in altering the electronic structure of the active site, narrowing the energy gap between Co 3d and O 2p orbitals, evidencing the enhancement of the M–O covalency. In addition, this increased M–O covalency accelerates charge transfer in oxygen-related reactions, thereby facilitating the reversible formation and decomposition of the discharge products in LOBs. As a proof of concept, the MnCr0.5Co1.5O4 catalyzed LOBs exhibit a large discharge capacity of 16 388.3 mAh g–1 and maintain stability over 329 cycles. This work paves the way for the progression of reversible LOBs by manipulating the coordination structure of the spinel catalysts.
调节钴基尖晶石八面体金属位的配位环境以提高金属-氧键共价性,从而实现可逆锂-氧电池
传统尖晶石电催化剂的内在催化活性阻碍了它们在锂-氧电池(LOB)中的电催化结果,尽管它们因成分多样性和结构适应性而具有吸引力。在这项研究中,我们发现通过在 MnCo2O4 八面体位点(MnCr0.5Co1.5O4)中引入 Cr 元素,调节金属-氧(M-O)键共价相互作用,可以从本质上增强这些催化剂的电催化活性。Cr3+ 的引入直接改变了 Co 八面体位点的配位结构,增加了 Co3+-O 间距,降低了晶格对称性,从而增强了 M-O 键的共价相互作用。计算分析证实了铬在改变活性位点电子结构方面的有效性,它缩小了 Co 3d 和 O 2p 轨道之间的能隙,证明了 M-O 共价作用的增强。此外,M-O 共价性的增强加快了氧相关反应中的电荷转移,从而促进了 LOB 中放电产物的可逆形成和分解。作为概念验证,MnCr0.5Co1.5O4 催化的 LOB 显示出 16 388.3 mAh g-1 的大放电容量,并在 329 次循环中保持稳定。这项工作为通过操纵尖晶石催化剂的配位结构来发展可逆 LOB 铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


