Sialylated IgG induces the transcription factor REST in alveolar macrophages to protect against lung inflammation and severe influenza disease
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
While most respiratory viral infections resolve with little harm to the host, severe symptoms arise when infection triggers an aberrant inflammatory response that damages lung tissue. Host regulators of virally induced lung inflammation have not been well defined. Here, we show that enrichment for sialylated, but not asialylated immunoglobulin G (IgG), predicted mild influenza disease in humans and was broadly protective against heterologous influenza viruses in a murine challenge model. Mechanistic studies show that sialylated IgG mediated this protection by inducing the transcription factor repressor element-1 silencing transcription factor (REST), which repressed nuclear factor κB (NF-κB)-driven responses, preventing severe lung inflammation and protecting lung function during influenza infection. Therapeutic administration of a recombinant, sialylated Fc molecule in clinical development similarly activated REST and protected against severe influenza disease, demonstrating that this pathway could be clinically harnessed. Overall, induction of REST through sialylated IgG signaling is a strategy to limit inflammatory disease sequelae in infections caused by antigenically distinct influenza strains.
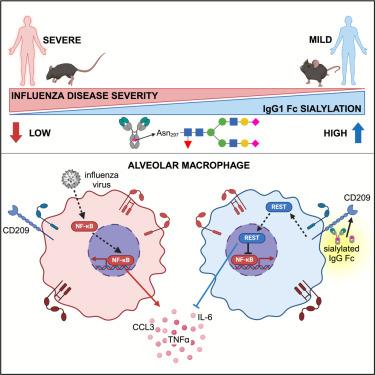
Sialylated IgG 可诱导肺泡巨噬细胞中的转录因子 REST,从而防止肺部炎症和严重流感疾病的发生
虽然大多数呼吸道病毒感染对宿主的伤害很小,但当感染引发异常炎症反应并损害肺组织时,就会出现严重症状。病毒诱导的肺部炎症的宿主调节因子尚未得到很好的定义。在这里,我们发现,富集硅氨酰化的免疫球蛋白 G(IgG)可预测人类的轻微流感疾病,而不富集淀粉酰化的免疫球蛋白 G(IgG)则可预测人类的轻微流感疾病,并且在小鼠挑战模型中对异源流感病毒具有广泛的保护作用。机理研究表明,硅氨酰化 IgG 通过诱导转录因子抑制因子-1 沉默转录因子 (REST),从而抑制核因子κB (NF-κB)驱动的反应,在流感感染期间防止严重的肺部炎症并保护肺功能。临床开发中的一种重组ialylated Fc分子的治疗用药同样激活了REST,并防止了严重流感疾病的发生,这表明这一途径可以在临床上加以利用。总之,在由抗原不同的流感病毒株引起的感染中,通过糖基化IgG信号诱导REST是一种限制炎症性疾病后遗症的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


