Environment-sensitive turn-on fluorescent probe enables live cell imaging of myeloperoxidase activity during NETosis
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Myeloperoxidase (MPO) plays an important role in the immune response of human neutrophils and has been implicated in autoimmune conditions, cardiovascular disorders, and neurodegeneration. Current methods to detect MPO activity rely on the detection of HOCl using activatable probes or require challenging experimental procedures. Therefore, these tools provide limited information about the dynamics and localization of MPO in complex molecular processes such as NETosis in real time. In this study, we report a ‘’turn-on” activity-based probe that fluoresces exclusively upon binding to MPO, exhibits minimal background fluorescence in buffered aqueous media, and is blocked by MPO inhibitors. Our probe facilitates real-time imaging of direct MPO activity in human neutrophils and HL-60-derived granulocytes during NETosis under wash-free conditions. Furthermore, it allows for the discrimination between different triggers of NETosis in human neutrophils. These findings hold promise for advancing our understanding of the role of MPO in immune responses and inflammatory conditions. Myeloperoxidase (MPO) plays an important role in the innate immune response of human neutrophils and has been implicated in various diseases, but current methods to detect MPO provide limited information about its dynamics and localization in complex molecular processes. Here, the authors develop an activity-based probe that fluoresces exclusively upon binding to MPO, enabling real-time imaging of direct intracellular MPO activity in human neutrophils and HL-60-derived granulocytes during NETosis under wash-free conditions.
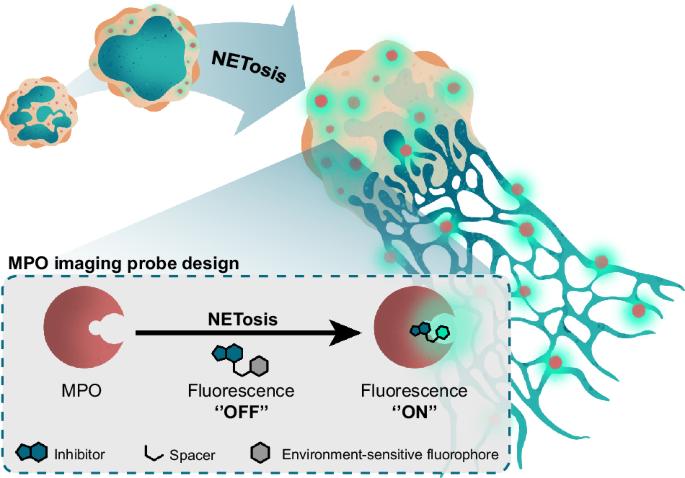
环境敏感型开启荧光探针可对母细胞瘤形成过程中髓过氧化物酶的活性进行活细胞成像
髓过氧化物酶(MPO)在人类中性粒细胞的免疫反应中发挥着重要作用,并与自身免疫性疾病、心血管疾病和神经变性有关。目前检测 MPO 活性的方法依赖于使用可激活探针检测 HOCl,或需要具有挑战性的实验程序。因此,这些工具提供的有关 MPO 在诸如 NETosis 等复杂分子过程中的动态和定位的实时信息非常有限。在这项研究中,我们报告了一种基于 "开启 "活性的探针,这种探针在与 MPO 结合时才会发出荧光,在缓冲水介质中的背景荧光极少,而且会被 MPO 抑制剂阻断。我们的探针有助于在免洗条件下对人中性粒细胞和 HL-60 衍生粒细胞在 NETosis 期间的直接 MPO 活性进行实时成像。此外,它还能区分人类中性粒细胞 NETosis 的不同诱因。这些发现有望加深我们对 MPO 在免疫反应和炎症中的作用的理解。髓过氧化物酶(MPO)在人类中性粒细胞的先天性免疫反应中发挥着重要作用,并与多种疾病有关,但目前检测 MPO 的方法只能提供有关其在复杂分子过程中的动态和定位的有限信息。在本文中,作者开发了一种基于活性的探针,该探针与 MPO 结合后会发出荧光,从而能在无冲洗条件下实时成像人中性粒细胞和 HL-60 衍生粒细胞在 NETosis 过程中细胞内 MPO 的直接活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


