Engineered Biomimetic Cancer Cell Membrane Nanosystems Trigger Gas-Immunometabolic Therapy for Spinal-Metastasized Tumors
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Despite great progress in enhancing tumor immunogenicity, conventional gas therapy cannot effectively reverse the tumor immunosuppressive microenvironment (TIME), limiting immunotherapy. The development of therapeutic gases that are tumor microenvironment responsive and efficiently reverse the TIME for precisely targeted tumor gas-immunometabolic therapy remains a great challenge. In this study, a novel cancer cell membrane-encapsulated pH-responsive nitric oxide (NO)-releasing biomimetic nanosystem (MP@AL) is prepared. Lactate oxidase (Lox) in MP@AL consumed oxygen to promote the decomposition of lactate, a metabolic by-product of tumor glycolysis, and the generation of H2O2, while L-arginine (L-Arg) in MP@AL is oxidized by H2O2 to generate nitric oxide (NO). For one thing, NO led to mitochondrial dysfunction in tumor cells to reduce oxygen consumption and promote the efficiency of Lox in lactate decomposition, thus reversing lactate-induced TIME; for another, NO effectively triggered immunogenic cell death, activated anti-tumor immune response and long-term immune memory, and ensured a favorable effect in the synergistic interaction with PD-L1 antibody for inhibiting tumor growth and recurrence. Therefore, a novel gas-immunometabolic therapy dual closed-loop nanosystem for enhancing tumor immunogenicity and remodeling lactate-induced TIME is established. Overall, this work will provide new ideas for gas therapy to effectively remodel the TIME to enhance cancer immunotherapy.
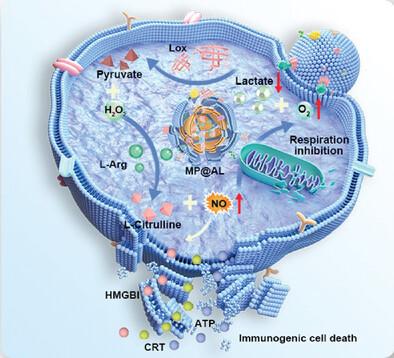
工程仿生癌细胞膜纳米系统触发脊柱转移肿瘤的气体-免疫代谢疗法
尽管在增强肿瘤免疫原性方面取得了巨大进展,但传统的气体疗法无法有效逆转肿瘤免疫抑制微环境(TIME),从而限制了免疫疗法。开发对肿瘤微环境有反应且能有效逆转肿瘤免疫抑制微环境(TIME)的治疗气体,实现精确靶向的肿瘤气体-免疫代谢疗法,仍然是一项巨大的挑战。本研究制备了一种新型癌细胞膜封装的 pH 响应型一氧化氮(NO)释放生物仿生纳米系统(MP@AL)。MP@AL中的乳酸氧化酶(Lox)消耗氧气促进肿瘤糖酵解代谢副产物乳酸的分解并生成H2O2,而MP@AL中的L-精氨酸(L-Arg)被H2O2氧化生成一氧化氮(NO)。一氧化氮导致肿瘤细胞线粒体功能障碍,降低耗氧量,促进Lox分解乳酸的效率,从而逆转乳酸诱导的TIME;二氧化氮有效引发免疫原性细胞死亡,激活抗肿瘤免疫反应和长期免疫记忆,确保与PD-L1抗体协同作用抑制肿瘤生长和复发的良好效果。因此,建立了一种新型气体-免疫代谢疗法双闭环纳米系统,用于增强肿瘤免疫原性和重塑乳酸诱导的 TIME。总之,这项工作将为气体疗法提供新的思路,以有效重塑乳酸诱导的 TIME,从而增强肿瘤免疫疗法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


