Design of electrocatalysts and electrodes for CO2 electroreduction to formic acid and formate
IF 20.3
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Electrochemical carbon dioxide (CO2) reduction reaction (CO2RR) is gaining attraction as it enables the generation of highly valuable chemicals while mitigating greenhouse gas emissions. Numerous efforts have been dedicated to designing efficient catalysts that selectively reduce CO2 into various chemicals such as formic acid/formate, carbon monoxide, methane, ethylene, and ethanol. The efficiency of the CO2RR is evaluated through selectivity to the desired product, overpotential, current density, and stability. This work reviews emerging strategies to improve the CO2RR performance to produce formate/formic acid by tuning catalyst and electrode structures. The catalyst-developing strategies are discussed in terms of engineering electronic and geometric structures, surface oxidation structure, and enlarging surface active area. The design of gas diffusion electrodes and reactor configuration, which are essential in enhancing the efficiency of the electrochemical system, are also mentioned. Finally, insights and perspectives are given on how to overcome the instability of catalysts and limitations of reactor designs.
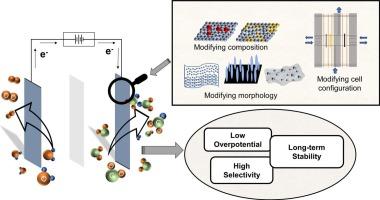
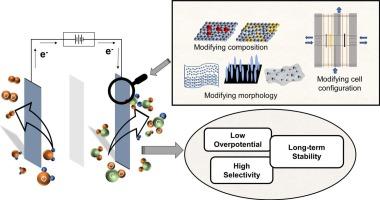
设计将二氧化碳电还原为甲酸和甲酸盐的电催化剂和电极
电化学二氧化碳(CO2)还原反应(CO2RR)在减少温室气体排放的同时,还能生成极具价值的化学品,因此越来越受到人们的青睐。人们一直致力于设计高效催化剂,将二氧化碳选择性地还原成甲酸/甲酸盐、一氧化碳、甲烷、乙烯和乙醇等各种化学品。CO2RR 的效率通过对所需产物的选择性、过电位、电流密度和稳定性进行评估。本研究综述了通过调整催化剂和电极结构提高 CO2RR 性能以生产甲酸/甲酸的新策略。催化剂开发策略从工程电子和几何结构、表面氧化结构以及扩大表面活性面积等方面进行了讨论。此外,还提到了对提高电化学系统效率至关重要的气体扩散电极和反应器配置的设计。最后,还就如何克服催化剂的不稳定性和反应器设计的局限性提出了见解和展望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Coordination Chemistry Reviews
化学-无机化学与核化学
CiteScore
34.30
自引率
5.30%
发文量
457
审稿时长
54 days
期刊介绍:
Coordination Chemistry Reviews offers rapid publication of review articles on current and significant topics in coordination chemistry, encompassing organometallic, supramolecular, theoretical, and bioinorganic chemistry. It also covers catalysis, materials chemistry, and metal-organic frameworks from a coordination chemistry perspective. Reviews summarize recent developments or discuss specific techniques, welcoming contributions from both established and emerging researchers.
The journal releases special issues on timely subjects, including those featuring contributions from specific regions or conferences. Occasional full-length book articles are also featured. Additionally, special volumes cover annual reviews of main group chemistry, transition metal group chemistry, and organometallic chemistry. These comprehensive reviews are vital resources for those engaged in coordination chemistry, further establishing Coordination Chemistry Reviews as a hub for insightful surveys in inorganic and physical inorganic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


