Shifting hydrogenation pathway via electronic activation for efficient nitrate electroreduction to ammonia in sewages
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Electrochemical hydrogenation reactions have attracted worldwide attention as a sustainable alternative to thermo-catalytic hydrogenations. Nevertheless, the Faradaic efficiency, in many cases, is limited by the competing side reaction of hydrogen evolution. In this work, we demonstrate that the hydrogenation pathway can be effectively modulated by electronic activation near the interface. In a heterostructure consisting of a Cu foam matrix and Co3O4 decoration layer (Co@Cu), the surface Co is effectively activated by electrons transferring from underneath Cu, leading to strongly promoted reactant adsorption and weakened Co-H bonding. Consequently, the hydrogenation pathway on the Co site shifts from H-H coupling to nitrate reduction, resulting in an outstanding nitrate reduction reaction (NO3−RR) Faradaic efficiency of 97.67%. A hybrid reactor combining electroreduction and membrane separation is further constructed to realize an NH3 recovery rate as high as 857.1 g-N m−2 d−1 from actual sewage. The results can be generalized for other electrochemical hydrogenation reactions for energy and environment applications.
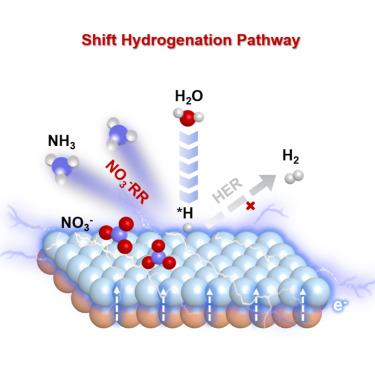
通过电子活化改变氢化途径,在污水中将硝酸盐高效电还原为氨气
电化学氢化反应作为热催化氢化反应的一种可持续替代反应,已经引起了全世界的关注。然而,在许多情况下,法拉第效率受到氢进化副反应的限制。在这项工作中,我们证明了氢化途径可以通过界面附近的电子活化进行有效调节。在一个由泡沫铜基体和 Co3O4 装饰层(Co@Cu)组成的异质结构中,表面 Co 被从铜下面转移过来的电子有效激活,从而强烈促进了反应物的吸附并削弱了 Co-H 键。因此,Co 位点上的氢化途径从 H-H 耦合转变为硝酸盐还原,从而使硝酸盐还原反应(NO3-RR)的法拉第效率高达 97.67%。进一步构建的电还原与膜分离相结合的混合反应器可从实际污水中实现高达 857.1 g-N m-2 d-1 的 NH3 回收率。研究结果可推广应用于能源和环境领域的其他电化学氢化反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


