Roles of Acidic Proton for Fe-Containing Zeolite in Direct Oxidation of Methane
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
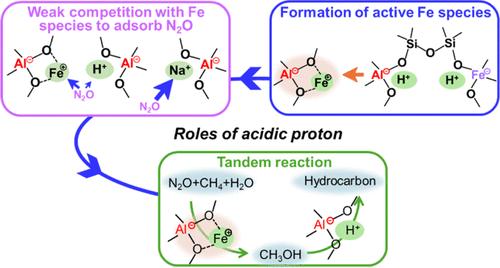
Fe-containing zeolite catalysts are active in N2O decomposition and direct oxidation of unreactive methane. Except for the well-known ability that acid sites realize the subsequent reaction of methanol to hydrocarbon, the roles of acidic protons in the direct oxidation of methane have not been studied regarding the formation of active Fe components and the reactivity in the reaction. Herein, on the premise of the comparable total Fe and Al contents, the acidity of one-pot synthesized Fe-AEI and ion-exchanged Fe/AEI zeolites was adjusted by various Na contents, and the catalytic activity in methane oxidation reactions was compared under different conditions. Ultraviolet–visible (UV–vis) spectra at 25–500 °C of the as-synthesized Fe-AEI and the reaction performance at the corresponding conditions were combined to clarify the formation of potential active Fe species. Acidic proton was favorable for the formation of potential active Fe species for the one-pot synthesized Fe-AEI zeolite. However, for the H-type Fe/AEI zeolite, thermal treatment at high temperatures was prone to dealuminate, reduced the number of anchors for Fe3+ attachment, and resulted in inactive FexOy. Furthermore, in contrast to the robust N2O adsorption capacity of Na+, the acidic proton exhibited weak competition with Fe3+ for N2O adsorption and thus contributed to the higher activity in the methane oxidation reaction. Our findings highlighted the importance of the acidic proton for the formation of potential active Fe species, the frail competition of adsorption N2O with Fe species, and the feasibility of the tandem reaction of methane to methanol and methanol to hydrocarbon.
含铁沸石的酸性质子在甲烷直接氧化中的作用
含铁沸石催化剂在分解 N2O 和直接氧化无活性甲烷方面具有活性。除了众所周知的酸性位点能实现甲醇到碳氢化合物的后续反应外,酸性质子在甲烷直接氧化中的作用还没有被研究过,即活性铁组分的形成和反应活性。本文在铁和铝总含量相当的前提下,通过不同的 Na 含量调节一锅合成的 Fe-AEI 和离子交换 Fe/AEI 沸石的酸度,比较了不同条件下甲烷氧化反应的催化活性。结合合成的 Fe-AEI 在 25-500 ℃ 的紫外可见光谱和相应条件下的反应性能,阐明了潜在活性铁物种的形成。对于一锅合成的 Fe-AEI 沸石,酸性质子有利于潜在活性铁物种的形成。然而,对于 H 型 Fe/AEI 沸石,高温热处理容易产生脱铝作用,减少了附着 Fe3+ 的锚的数量,导致 FexOy 失去活性。此外,与 Na+ 强大的 N2O 吸附能力相反,酸性质子在 N2O 吸附方面与 Fe3+ 的竞争很弱,因此在甲烷氧化反应中具有更高的活性。我们的研究结果突显了酸性质子对形成潜在活性 Fe 物种的重要性、吸附 N2O 与 Fe 物种的微弱竞争性以及甲烷制甲醇和甲醇制碳氢化合物串联反应的可行性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


