A β-hydroxybutyrate shunt pathway generates anti-obesity ketone metabolites
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
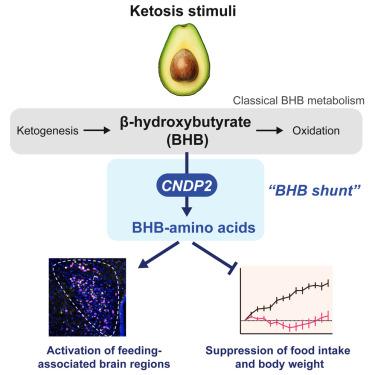
β-Hydroxybutyrate (BHB) is an abundant ketone body. To date, all known pathways of BHB metabolism involve the interconversion of BHB and primary energy intermediates. Here, we identify a previously undescribed BHB secondary metabolic pathway via CNDP2-dependent enzymatic conjugation of BHB and free amino acids. This BHB shunt pathway generates a family of anti-obesity ketone metabolites, the BHB-amino acids. Genetic ablation of CNDP2 in mice eliminates tissue amino acid BHB-ylation activity and reduces BHB-amino acid levels. The most abundant BHB-amino acid, BHB-Phe, is a ketosis-inducible congener of Lac-Phe that activates hypothalamic and brainstem neurons and suppresses feeding. Conversely, CNDP2-KO mice exhibit increased food intake and body weight following exogenous ketone ester supplementation or a ketogenic diet. CNDP2-dependent amino acid BHB-ylation and BHB-amino acid metabolites are also conserved in humans. Therefore, enzymatic amino acid BHB-ylation defines a ketone shunt pathway and bioactive ketone metabolites linked to energy balance.
β-羟丁酸分流途径产生抗肥胖酮体代谢物
β-羟丁酸(BHB)是一种丰富的酮体。迄今为止,所有已知的 BHB 代谢途径都涉及 BHB 和初级能量中间产物的相互转化。在这里,我们通过 BHB 和游离氨基酸的 CNDP2 依赖性酶促共轭作用,发现了一种以前未曾描述过的 BHB 二级代谢途径。这种 BHB 分流途径产生了一系列抗肥胖酮类代谢物--BHB-氨基酸。对小鼠进行 CNDP2 基因消减可消除组织氨基酸的 BHB-酰化活性,并降低 BHB-氨基酸的水平。最丰富的 BHB-氨基酸 BHB-Phe 是一种酮病诱导的 Lac-Phe 同系物,可激活下丘脑和脑干神经元并抑制摄食。相反,CNDP2-KO 小鼠在补充外源性酮酯或摄入生酮饮食后,食物摄入量和体重都会增加。依赖于 CNDP2 的氨基酸 BHB-酰化和 BHB-氨基酸代谢产物在人类中也是保守的。因此,氨基酸 BHB-酰化酶定义了酮分流途径和与能量平衡相关的生物活性酮代谢物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


