Post-transplant G-CSF impedes engraftment of gene-edited human hematopoietic stem cells by exacerbating p53-mediated DNA damage response
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
Granulocyte-colony-stimulating factor (G-CSF) is commonly used to accelerate recovery from neutropenia following chemotherapy and autologous transplantation of hematopoietic stem and progenitor cells (HSPCs) for malignant disorders. However, its utility after ex vivo gene therapy in human HSPCs remains unexplored. We show that administering G-CSF from day 1 to 14 post-transplant impedes engraftment of CRISPR-Cas9 gene-edited human HSPCs in murine xenograft models. G-CSF affects gene-edited HSPCs through a cell-intrinsic mechanism, causing proliferative stress and amplifying the early p53-mediated DNA damage response triggered by Cas9-mediated DNA double-strand breaks. This underscores a threshold mechanism where p53 activation must reach a critical level to impair cellular function. Transiently inhibiting p53 or delaying the initiation of G-CSF treatment to day 5 post-transplant attenuates its negative impact on gene-edited HSPCs. The potential for increased HSPC toxicity associated with post-transplant G-CSF administration in CRISPR-Cas9 autologous HSPC gene therapy warrants consideration in clinical trials.
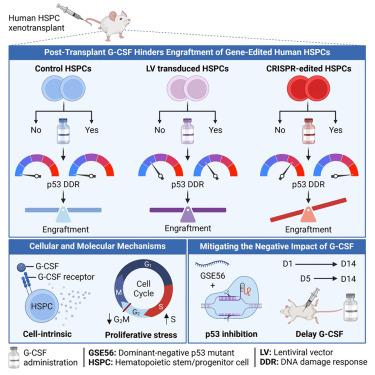
移植后的 G-CSF 通过加剧 p53 介导的 DNA 损伤反应,阻碍基因编辑的人类造血干细胞的移植
粒细胞-淋巴细胞刺激因子(G-CSF)常用于加速化疗后中性粒细胞减少症的恢复,以及自体移植造血干细胞和祖细胞(HSPCs)治疗恶性疾病。然而,它在人类 HSPCs 体外基因治疗后的效用仍有待探索。我们的研究表明,在小鼠异种移植模型中,移植后第1到14天注射G-CSF会阻碍CRISPR-Cas9基因编辑的人类HSPC的移植。G-CSF通过细胞内在机制影响基因编辑的HSPCs,导致增殖应激,并扩大由Cas9介导的DNA双链断裂引发的早期p53介导的DNA损伤反应。这强调了一种阈值机制,即 p53 激活必须达到临界水平才能损害细胞功能。瞬时抑制 p53 或将 G-CSF 治疗推迟到移植后第 5 天,可减轻其对基因编辑 HSPC 的负面影响。在 CRISPR-Cas9 自体 HSPC 基因疗法中,移植后 G-CSF 给药可能会增加 HSPC 的毒性,这值得在临床试验中加以考虑。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


