Superior Photostability of the Unnatural Base 6-Amino-5-nitropyridin-2-ol: A Case Study Using Ultrafast Broadband Fluorescence, Transient Absorption, and Theoretical Computation
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
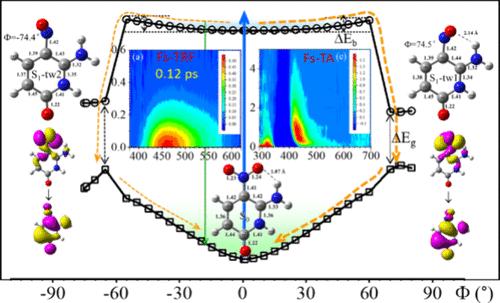
6-Amino-5-nitropyridin-2-ol (Z), a nitroaromatic compound and a base for Hachimoji nucleic acids, holds significant potential in expanding the genetic alphabet, as well as in synthetic biology and biotechnology. Despite its promising applications, the spectral characterization and photoinduced properties of Z have remained largely unexplored until now. This study presents a comprehensive investigation into its excited state dynamics in various solvents, utilizing state-of-the-art ultrafast broadband time-resolved fluorescence and transient absorption spectroscopy, complemented by computational methods. The acquired results provide direct experimental evidence that, upon photoexcitation, Z emits prompt fluorescence from a nearly planar structure in its excited state, independent of solvent properties. This state deactivates nonradiatively within sub-picoseconds through internal conversion with a unitary yield, primarily mediated by the rotation of the nitro group. This unusually rapid deactivation pathway entirely excludes the involvement of long-lived nπ* states, triplet states, and photoproducts, which are commonly observed in most nitroaromatic compounds and natural DNA and RNA bases. Our findings underscore that Z, as an unnatural base, exhibits superior photostability compared to canonical natural bases. This provides valuable insights into the photodynamics of nitroaromatic compounds, which is beneficial for strategic substitution design in environmental and biological applications.
非天然碱 6-氨基-5-硝基吡啶-2-醇的卓越光稳定性:利用超快宽带荧光、瞬态吸收和理论计算进行的案例研究
6- 氨基-5-硝基吡啶-2-醇(Z)是一种硝基芳香族化合物,也是八嘧啶核酸的基质,在扩展遗传字母表以及合成生物学和生物技术方面具有巨大潜力。尽管其应用前景广阔,但到目前为止,Z 的光谱特征和光诱导特性在很大程度上仍未得到研究。本研究利用最先进的超快宽带时间分辨荧光光谱和瞬态吸收光谱,并辅以计算方法,对 Z 在各种溶剂中的激发态动力学进行了全面研究。所获得的结果提供了直接的实验证据,证明 Z 在激发态时会从一个近乎平面的结构中迅速发出荧光,与溶剂特性无关。这种状态主要通过硝基的旋转,在亚皮秒内通过内部转换以非辐射的方式失活,并产生单位产量。这种异常快速的失活途径完全排除了长寿命 nπ* 态、三重态和光产物的参与,而这些在大多数硝基芳香族化合物以及天然 DNA 和 RNA 碱基中都很常见。我们的研究结果表明,与典型的天然碱基相比,Z 作为一种非天然碱基,具有更优越的光稳定性。这为我们深入了解硝基芳香族化合物的光动力学提供了宝贵的资料,有利于在环境和生物应用中进行战略性替代设计。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


