Vibrational Relaxation Completes the Excitation Energy Transfer and Localization of Vibronic Excitons in Allophycocyanin α84-β84
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Phycobilisomes are light-harvesting complexes that play a key role in photosynthesis in cyanobacteria, which generate more than 40% of the world’s oxygen. The near-unity excitation energy transfer efficiency from phycobilisomes to photosystems highlights its importance in understanding efficient energy transfer processes. Spectroscopic studies have shown that the 280 fs rapid excitonic downhill energy transfer within the α84-β84 chromophore dimer in allophycocyanin (APC), a subunit of phycobilisomes, is crucial to this efficiency. However, the role of strong chromophore–protein interactions and vibrational relaxation requires further exploration to fully explain this efficient downhill energy transfer. A theory is required that adequately describes exciton dynamics in an intermediate region while also incorporating vibrational relaxation mediated by protein bath modes. In this work, we incorporate vibrational relaxation into modified Redfield theory by introducing coupling fluctuation. We holistically simulate the rapid excitation energy transfer process of the α84-β84 chromophore dimer in APC and successfully model the recently observed rapid energy capture. We find that vibrational relaxation dictates capture of excitons by the localized state of the β84 chromophore. The calculated rate shows excellent agreement with previous ultrafast spectroscopic experiments. Our results show that the inclusion of vibrational relaxation is essential for systems that utilize vibronic coupling to enhance energy transfer and capture. Consequently, incorporating vibrational relaxation into Modified Redfield theory shows promise for accurately describing the excitation energy transfer process in other photosynthetic systems.
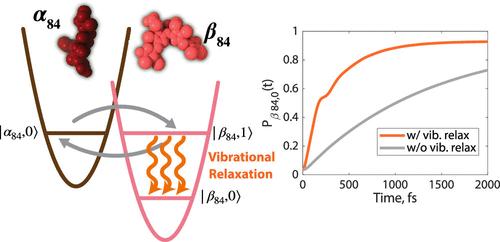
振动弛豫完成异叶花青素 α84-β84 中振动激子的激发能量转移和定位
藻青体是一种光收集复合体,在蓝藻的光合作用中发挥着关键作用,蓝藻产生的氧气占全球总量的40%以上。从藻体到光系统的近乎统一的激发能量传递效率突显了它在了解高效能量传递过程中的重要性。光谱研究表明,藻青体亚基异叶花青素(APC)中α84-β84发色团二聚体内280 fs的快速激子下坡能量转移对这种效率至关重要。然而,要充分解释这种高效的下坡能量转移,还需要进一步探讨发色团与蛋白质之间的强相互作用和振动弛豫的作用。我们需要一种理论,既能充分描述中间区域的激子动力学,又能纳入由蛋白质浴模式介导的振动弛豫。在这项工作中,我们通过引入耦合波动,将振动弛豫纳入修正的雷德菲尔德理论。我们全面模拟了 APC 中 α84-β84 发色团二聚体的快速激发能量转移过程,并成功模拟了最近观测到的快速能量捕获。我们发现振动弛豫决定了 β84 发色团局部态对激子的捕获。计算出的速率与之前的超快光谱实验结果非常吻合。我们的研究结果表明,对于利用振子耦合来增强能量转移和捕获的系统来说,加入振动弛豫是必不可少的。因此,将振动弛豫纳入修正雷德菲尔德理论有望准确描述其他光合作用系统的激发能量转移过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


