Resonant Vibrational Enhancement of Downhill Energy Transfer in the C-Phycocyanin Chromophore Dimer
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Energy transfer between electronically coupled photosynthetic light-harvesting antenna pigments is frequently assisted by protein and chromophore nuclear motion. This energy transfer mechanism usually occurs in the weak or intermediate system-bath coupling regime. Redfield theory is frequently used to describe the energy transfer in this regime. Spectral densities describe vibronic coupling in visible transitions of the chromophores and govern energy transfer in the Redfield mechanism. In this work, we perform finely sampled broadband pump–probe spectroscopy on the phycobilisome antenna complex with sub-10-fs pump and probe pulses. The spectral density obtained by Fourier transforming the pump–probe time-domain signal is used to perform modified Redfield rate calculations to check for vibrational enhancement of energy transfer in a coupled chromophore dimer in the C-phycocyanin protein of the phycobilisome antenna. We find two low-frequency vibrations to be in near-resonance with the interexcitonic energy gap and a few-fold enhancement in the interexcitonic energy transfer rate due to these resonances at room temperature. Our observations and calculations explain the fast downhill energy transfer process in C-phycocyanin. We also observe high-frequency vibrations involving chromophore–protein residue interactions in the excited state of the phycocyanobilin chromophore. We suggest that these vibrations lock the chromophore nuclear configuration of the excited state and prevent the energetic relaxation that blocks energy transfer.
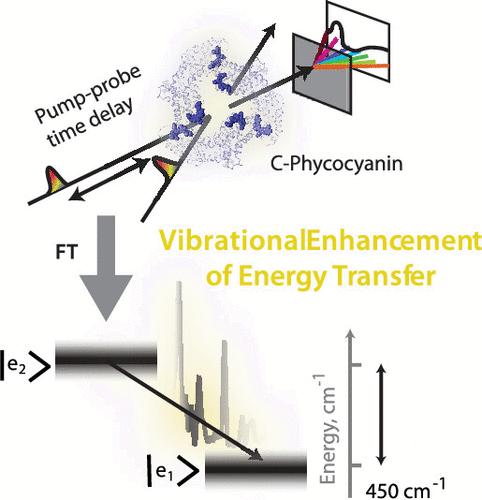
C-Phycocyanin 色素二聚体中下坡能量转移的共振振动增强
电子耦合光合作用光收集天线色素之间的能量转移通常由蛋白质和发色团的核运动辅助。这种能量传递机制通常发生在弱或中间系统-浴耦合机制中。Redfield 理论常用于描述这种机制下的能量传递。光谱密度描述了发色团可见光跃迁中的振子耦合,并支配着 Redfield 机制中的能量转移。在这项工作中,我们用亚 10-fs 泵脉冲和探针脉冲对藻体天线复合物进行了精细采样的宽带泵-探针光谱分析。通过对泵浦-探针时域信号进行傅里叶变换得到的光谱密度被用来进行修正的雷德菲尔德速率计算,以检查藻青体天线的 C-phycocyanin蛋白质中的耦合发色团二聚体是否存在振动增强能量传递的现象。我们发现有两个低频振动与放电内能隙接近共振,并且由于这些共振,放电内能量传递速率在室温下提高了几倍。我们的观察和计算解释了 C-植物花青素的快速下坡能量转移过程。我们还观察到藻蓝蛋白发色团激发态中涉及发色团与蛋白质残基相互作用的高频振动。我们认为,这些振动锁定了激发态的发色团核构型,阻止了阻碍能量传递的能量弛豫。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


