DNA damage response inhibitors in cancer therapy: lessons from the past, current status and future implications
IF 122.7
1区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
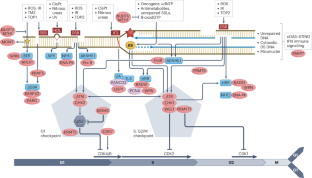
The DNA damage response (DDR) is a network of proteins that coordinate DNA repair and cell-cycle checkpoints to prevent damage being transmitted to daughter cells. DDR defects lead to genomic instability, which enables tumour development, but they also create vulnerabilities that can be used for cancer therapy. Historically, this vulnerability has been taken advantage of using DNA-damaging cytotoxic drugs and radiotherapy, which are more toxic to tumour cells than to normal tissues. However, the discovery of the unique sensitivity of tumours defective in the homologous recombination DNA repair pathway to PARP inhibition led to the approval of six PARP inhibitors worldwide and to a focus on making use of DDR defects through the development of other DDR-targeting drugs. Here, we analyse the lessons learnt from PARP inhibitor development and how these may be applied to new targets to maximize success. We explore why, despite so much research, no other DDR inhibitor class has been approved, and only a handful have advanced to later-stage clinical trials. We discuss why more reliable predictive biomarkers are needed, explore study design from past and current trials, and suggest alternative models for monotherapy and combination studies. Targeting multiple DDR pathways simultaneously and potential combinations with anti-angiogenic agents or immune checkpoint inhibitors are also discussed. Defects in the DNA damage response have been utilized therapeutically for cancer for a decade. This Review analyses the lessons learnt from the development of PARP inhibitors and how these may be applied to new targets to maximize success. Targeting multiple DNA damage response pathways simultaneously and combinations with other therapies are also discussed.

癌症治疗中的 DNA 损伤反应抑制剂:过去的教训、现状和未来的影响
DNA 损伤应答(DDR)是一个蛋白质网络,它协调 DNA 修复和细胞周期检查点,防止损伤传递给子细胞。DDR 缺陷会导致基因组不稳定,使肿瘤得以发展,但同时也会产生可用于癌症治疗的漏洞。一直以来,人们都是利用这种弱点,使用对 DNA 有破坏作用的细胞毒性药物和放射疗法,因为它们对肿瘤细胞的毒性比对正常组织的毒性更大。然而,同源重组DNA修复途径缺陷的肿瘤对PARP抑制具有独特的敏感性,这一发现促使全球批准了六种PARP抑制剂,并促使人们通过开发其他DDR靶向药物来重点利用DDR缺陷。在此,我们分析了从 PARP 抑制剂开发中汲取的经验教训,以及如何将这些经验教训应用于新靶点以取得最大成功。我们探讨了为什么尽管进行了如此多的研究,但仍没有其他 DDR 抑制剂类药物获得批准,只有少数几种药物进入了后期临床试验阶段。我们讨论了为什么需要更可靠的预测性生物标志物,探讨了过去和当前试验的研究设计,并提出了单药治疗和联合研究的替代模式。我们还讨论了同时靶向多种 DDR 通路以及与抗血管生成药物或免疫检查点抑制剂的潜在组合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews. Drug Discovery
医学-生物工程与应用微生物
CiteScore
137.40
自引率
0.30%
发文量
227
期刊介绍:
Nature Reviews Drug Discovery is a monthly journal aimed at everyone working in the drug discovery and development arena.
Each issue includes:
Highest-quality reviews and perspectives covering a broad scope.
News stories investigating the hottest topics in drug discovery.
Timely summaries of key primary research papers.
Concise updates on the latest advances in areas such as new drug approvals, patent law, and emerging industry trends and strategies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


