Step-Economic Synthesis of Tamsulosin Hydrochloride via Continuous Chlorosulfonation and Biocatalytic Transamination
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
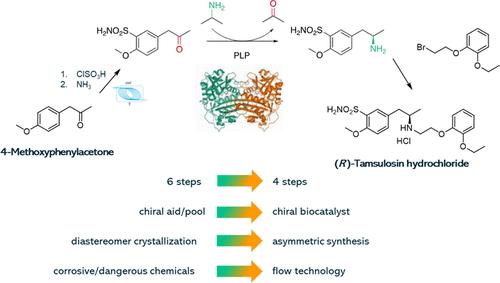
A new process was developed for the synthesis of (R)-tamsulosin in 4 chemical steps from readily available 4-methoxyphenylacetone using continuous chlorosulfonation and biocatalysis. Several conditions were tested for both batch and continuous chlorosulfonation of 4-methoxyphenylacetone. Continuous chlorosulfonation produced a white crystalline solid, while a brown solid or dark oil was consistently obtained when the reaction was performed in batch. Consequently, the sulfonamide intermediate was isolated as a white product in the continuous process, albeit in a slightly lower yield. Immobilized Escherichia coli whole cells overexpressing (R)-selective transaminases from Arthrobacter sp. (ArR-ATA and ArRmut-ATA, natural and engineered, respectively) and Aspergillus terreus (AtR-ATA), along with lyophilized amine transaminase (ATAs), were screened for the key asymmetric synthesis of the chiral amine intermediate. Under optimal conditions, conversions above 90% with >99% enantiomeric excess (ee) were achieved. Furthermore, for process intensification purposes, ATA-412 was covalently immobilized onto surface-activated mesoporous methacrylate beads, achieving quantitative immobilization yields. Immobilization and transamination were scaled up 30-fold, and the synthesized chiral amine intermediate was subjected to N-alkylation without isolation, yielding (R)-tamsulosin hydrochloride. Therefore, after scale-up, this synthesis shows a high potential to replace the current manufacturing process.
通过连续氯磺化和生物催化反式转化逐步经济地合成盐酸坦索罗辛
利用连续氯磺化和生物催化技术,开发了一种新工艺,可通过 4 个化学步骤从容易获得的 4-甲氧基苯丙酮合成 (R)-坦索罗辛。对 4-甲氧基苯丙酮的间歇和连续氯磺化进行了多种条件测试。连续氯磺化反应生成的是白色结晶固体,而间歇反应生成的则一直是棕色固体或深色油。因此,在连续式工艺中,磺酰胺中间体以白色产物的形式分离出来,尽管产率略低。筛选了过表达来自节杆菌(ArR-ATA 和 ArRmut-ATA,分别为天然和工程)和赤曲霉(AtR-ATA)的 (R) 选择性转氨酶的固定化大肠杆菌全细胞,以及冻干胺转氨酶 (ATA),用于手性胺中间体的关键不对称合成。在最佳条件下,转化率超过 90%,对映体过量率达 99%。此外,为了强化工艺,ATA-412 被共价固定在表面活性介孔甲基丙烯酸酯珠上,实现了定量固定化收率。固定化和转氨作用放大了 30 倍,合成的手性胺中间体在不分离的情况下进行了 N-烷基化,得到了盐酸(R)-坦索罗辛。因此,经过放大,该合成方法极有可能取代目前的生产工艺。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


