Proximal Anchoring of Nanodrugs through In Situ Generated Radical Hooks with Boosted Autophagy and Immunotherapy
IF 9.6
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Efficient retention of drugs at tumor sites was always desirable to maximize therapeutic functions, yet the main concern is the dynamic blood clearance induced fast removal from localized lesion. Herein, a tumor microenvironment activated covalently conjugation (self- and proximal conjugation) of tyramine modified Pt nanoclusters (PCMT NPs) was constructed by in situ produced radical hooks, leading to efficient accumulation of PCMT NPs at tumor sites. Such accumulation further aggravated the oxidative stress and provoked autophagy of tumor cells via activating the caspase-3 pathway mediated massive apoptosis, thereby stimulating immunogenic cell death (ICD). As verified by in vivo results, the PCMT NPs effectively suppressed primary and distant tumor growth (with an inhibition rate of 99%) while eliciting immunotherapeutic responses. As such, a new paradigm for boosting drug retention was provided, which enabled specific tumor treatment with synergistic therapeutic outcomes.
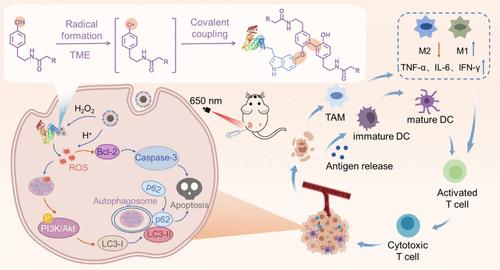
通过原位生成的自由基钩子近端锚定纳米药物,促进自噬和免疫疗法
为了最大限度地发挥治疗功能,药物在肿瘤部位的高效保留一直是人们所期望的,但主要的问题是动态血液清除引起的局部病灶的快速清除。本文通过原位产生的自由基钩子构建了一种肿瘤微环境激活的共价结合(自结合和近端结合)的酪胺修饰铂纳米团簇(PCMT NPs),从而使 PCMT NPs 在肿瘤部位有效聚集。这种积聚进一步加剧了氧化应激,并通过激活 Caspase-3 通路介导的大量细胞凋亡来诱发肿瘤细胞的自噬,从而刺激免疫原性细胞死亡(ICD)。体内实验结果证实,PCMT NPs 能有效抑制原发性和远处肿瘤的生长(抑制率达 99%),同时激发免疫治疗反应。因此,这为提高药物保留率提供了一种新的范例,从而实现了具有协同治疗效果的特异性肿瘤治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


