Palladium-assisted NOx storage and release on CexZr1-xO2 for passive NOx adsorber in diesel exhaust aftertreatment
引用次数: 0
Abstract
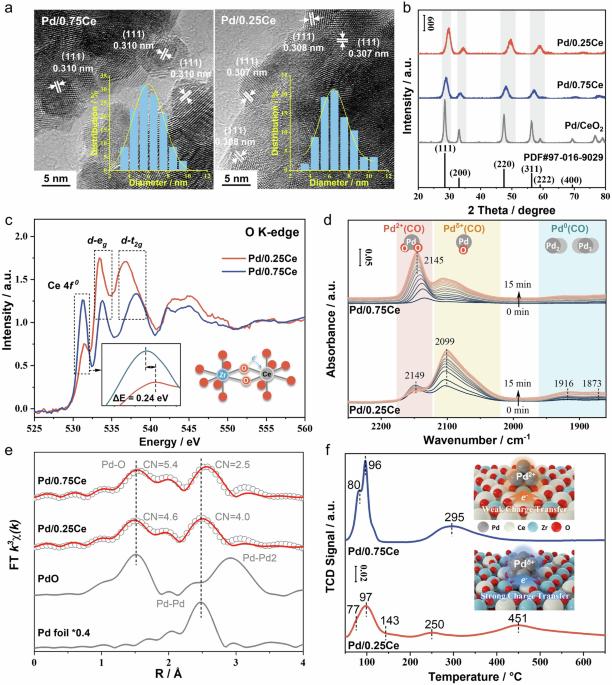
Understanding Pd effects on NOx storage and release is crucial for designing passive NOx adsorber (PNA) to control NOx emissions during diesel cold-starts. Herein, we report two oxidation states of Pd species on CexZr1-xO2 regulated by metal-support interaction. Pdδ+ (0 < δ < 2) in Pd/Ce0.25Zr0.75O2 exhibits a high affinity for O2 adsorption, which promotes the oxidation of adsorbed NO to nitrates at 100 °C. These nitrates are thermally unstable due to electron transfer from the Pd atom to the N-O bond, facilitating the decomposition of nitrates to NO2 above 200 °C. In contrast, Pd2+ in Pd/Ce0.75Zr0.25O2 prefer to NO adsorption. A large amount of adsorbed NO and nitrites accumulate on Pd2+ and Ce4+ results in high levels of NO release below 200 °C. For the potential application in PNA, Pd/Ce0.25Zr0.75O2 is recommended due to its proper NOx release temperature as well as better water and SO2 resistance. Pd-exchanged zeolites are widely studied as passive NOx adsorber materials for emission control for combustion engines. Here, Yue Peng and colleagues reveal that a relatively strong metal-support interaction between Pd and Ce0.25Zr0.75O2 leads to two oxidation states of Pd optimizing NOx storage efficiency and NOx release temperature.
钯辅助氮氧化物在 CexZr1-xO2 上的储存和释放,用于柴油机尾气后处理中的被动式氮氧化物吸附器
了解钯对氮氧化物储存和释放的影响对于设计被动式氮氧化物吸附器(PNA)以控制柴油机冷启动时的氮氧化物排放至关重要。在此,我们报告了 CexZr1-xO2 上受金属-支撑相互作用调控的两种钯氧化态。Pd/Ce0.25Zr0.75O2 中的 Pdδ+ (0 < δ < 2) 对 O2 具有很高的吸附亲和力,这促进了吸附的 NO 在 100 °C 下氧化成硝酸盐。由于电子从 Pd 原子转移到 N-O 键,这些硝酸盐具有热不稳定性,从而促进硝酸盐在 200 °C 以上分解为 NO2。相比之下,Pd/Ce0.75Zr0.25O2 中的 Pd2+ 更倾向于吸附 NO。大量吸附的 NO 和亚硝酸盐在 Pd2+ 和 Ce4+ 上积累,导致大量 NO 在 200 °C 以下释放。由于 Pd/Ce0.25Zr0.75O2 具有适当的氮氧化物释放温度以及更好的耐水性和耐二氧化硫性,因此建议将其用于 PNA 中。作为内燃机排放控制的被动氮氧化物吸附材料,Pd 交换沸石被广泛研究。岳鹏及其同事在此揭示了 Pd 与 Ce0.25Zr0.75O2 之间相对较强的金属-支撑相互作用会导致 Pd 的两种氧化态,从而优化氮氧化物存储效率和氮氧化物释放温度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


