Stability and magnetic properties of transition metal (V, Cr, Mn, and Fe) doped cobalt oxide clusters: a density functional theory investigation†
IF 3.9
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Con−1TMOn−2+ (n = 6–8), (TM = V, Cr, Mn, and Fe) clusters are investigated using density functional theory calculations. The transition metal atoms preferentially replace one Co atom at sites where the number of metal–oxygen bonds is maximized, forming more stable structures. The evaporation of a Co atom is the most fragile dissociation channel for both pure and doped species. Bare cobalt oxide clusters exhibit parallel spin ordering, whereas both parallel and antiparallel spin ordering are observed in the doped species. Notably, a ferromagnetic-to-ferrimagnetic transition occurs in the V-doped clusters, while the ferromagnetic behavior is enhanced in the Fe-doped species.
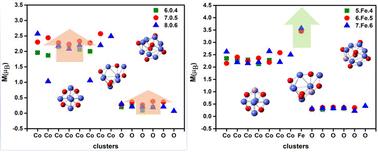
掺杂过渡金属(V、Cr、Mn 和 Fe)的氧化钴团簇的稳定性和磁性:密度泛函理论研究†。
利用密度泛函理论计算研究了 Con-1TMOn-2+(n = 6-8)(TM = V、Cr、Mn 和 Fe)团簇。过渡金属原子优先在金属氧键数量最大的位置取代一个 Co 原子,从而形成更稳定的结构。对于纯钴和掺杂钴来说,一个钴原子的蒸发是最脆弱的解离通道。裸氧化钴团簇表现出平行自旋排序,而在掺杂物种中则观察到平行和反平行自旋排序。值得注意的是,掺杂 V 的氧化钴团簇发生了从铁磁性到铁磁性的转变,而掺杂 Fe 的氧化钴团簇则增强了铁磁性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

RSC Advances
chemical sciences-
CiteScore
7.50
自引率
2.60%
发文量
3116
审稿时长
1.6 months
期刊介绍:
An international, peer-reviewed journal covering all of the chemical sciences, including multidisciplinary and emerging areas. RSC Advances is a gold open access journal allowing researchers free access to research articles, and offering an affordable open access publishing option for authors around the world.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


