A safe and efficient synthesis of N-Boc-β3-amino acid methyl esters from α-amino acids: applications in the formal synthesis of sedum alkaloids†
IF 3.9
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
β3-Amino acids are essential components in the synthesis of biologically active compounds. However, obtaining them in enantiomerically pure forms remains challenging. This study investigates a safe and efficient method for synthesizing enantiopure N-Boc-β3-amino acid methyl esters, incorporating both natural and unnatural side chains. The procedure avoids the use of expensive and toxic reagents, providing a safer alternative to the hazardous Arndt–Eistert homologation and cyanation reactions, which typically begin with enantiopure α-amino acids. The practical value of this transformation was demonstrated in the formal synthesis of sedum alkaloids.
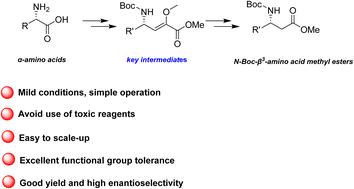
从α-氨基酸安全高效地合成 N-叔丁氧羰基-β3-氨基酸甲酯:在沉香生物碱正式合成中的应用†。
β3-氨基酸是合成生物活性化合物的重要成分。然而,获得对映体纯度高的氨基酸仍具有挑战性。本研究探讨了一种安全高效的方法,用于合成含有天然和非天然侧链的对映体纯 N-叔丁氧羰基-β3-氨基酸甲酯。该方法避免了使用昂贵且有毒的试剂,为危险的 Arndt-Eistert 同源反应和氰化反应提供了更安全的替代方案,后者通常以对映体α-氨基酸为起始原料。这种转化的实用价值已在沉香生物碱的正式合成中得到证明。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

RSC Advances
chemical sciences-
CiteScore
7.50
自引率
2.60%
发文量
3116
审稿时长
1.6 months
期刊介绍:
An international, peer-reviewed journal covering all of the chemical sciences, including multidisciplinary and emerging areas. RSC Advances is a gold open access journal allowing researchers free access to research articles, and offering an affordable open access publishing option for authors around the world.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


