Regulatable selective synthesis of benzofurans and coumarins from aryl propargyl ethers via an electrochemical tandem cyclization reaction†
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
A divergent protocol for the selective synthesis of benzofuran-2-carboxaldehydes and 3-organoselenyl-2H-coumarins from propargylic aryl ethers in the presence of dialkyl(aryl) diselenides under electrochemical reaction conditions was established. By adjusting the reactant ratio, reaction time and temperature, two significant heterocyclic derivatives could be selectively obtained with high yields, respectively. The reaction was performed under simple reaction conditions and was suitable for various substituted propargylic aryl ethers and dialkyl(aryl) diselenides.
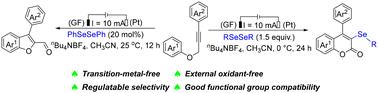
通过电化学串联环化反应从芳基丙炔醚中选择性地合成苯并呋喃和香豆素
在电化学反应条件下,在二烷基(芳基)二硒化物存在下,建立了从丙炔基芳基醚选择性合成苯并呋喃-2-甲醛和 3-有机硒基-2H-香豆素的不同方案。通过调节反应物比例、反应时间和温度,可分别选择性地获得两种重要的杂环衍生物,且产率较高。该反应在简单的反应条件下进行,适用于各种取代的丙炔基芳基醚和二烷基(芳基)二硒化物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


