Fe-Doped Ni-Based Catalysts Surpass Ir-Baselines for Oxygen Evolution Due to Optimal Charge-Transfer Characteristics
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
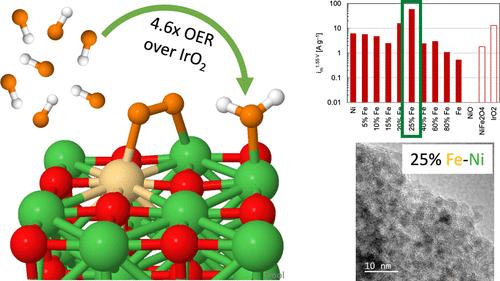
Ni-based catalysts with Co or Fe can potentially replace precious Ir-based catalysts for the rate-limiting oxygen evolution reaction (OER) in anion-exchange membrane (AEM) electrolyzers. In this study, density functional theory (DFT) calculations provide atomic- and electronic-level resolution on how the inclusion of Co or Fe can overcome the inactivity of NiO catalysts and even enable them to surpass IrO2 in activating key steps to the OER. Namely, NiO resists binding the key OH* intermediate and presents a high energetic barrier to forming the O*. Co- and Fe-substitution of Ni active sites allows for the stronger binding of OH* and preferentially activates O*/O2* formation, with Fe-substitution increasing the OER activity substantially as compared to Co-substitution. Whereas IrO2 requires an activation energy of 0.34–0.49 eV to form O2, this step is spontaneous on Fesub-NiO. Electrodeposition of polycrystalline electrodes and synthesized nanoparticles exploit the Co or Fe presence, with Fe particularly exhibiting greater activity: Tafel slopes indicate a significant change in the mechanism as compared to pure NiO, validating the theoretical predictions of OER activation at different steps. High-performing synthesized nanoparticles of 25% Fe–Ni exhibited a 4.6 times improvement over IrO2 and a 34% improvement over RuO2, showcasing that non-platinum group metal catalysts can outperform platinum group metals. High-resolution transmission electron microscopy further highlights the advantages of Fe–Ni oxide synthesized nanoparticles over commercial catalysts: small, randomly oriented nanoparticles expose greater edge sites than large nanoparticles typical of commercially available materials.
掺杂铁的镍基催化剂因其最佳电荷转移特性而在氧进化方面超越了铱基催化剂
含有 Co 或 Fe 的镍基催化剂有可能取代珍贵的 Ir 基催化剂,用于阴离子交换膜(AEM)电解器中的限速氧进化反应(OER)。在这项研究中,密度泛函理论(DFT)计算提供了原子和电子层面的解析,说明加入 Co 或 Fe 如何克服 NiO 催化剂的不活泼性,甚至使其在激活 OER 关键步骤方面超过 IrO2。也就是说,NiO 无法与关键的 OH* 中间体结合,并且在形成 O* 时具有很高的能量障碍。镍活性位点的共取代和铁取代可以更强地结合 OH*,并优先激活 O*/O2*的形成,与共取代相比,铁取代大大提高了 OER 活性。IrO2 需要 0.34-0.49 eV 的活化能才能形成 O2,而在 Fesub-NiO 上这一步骤是自发的。多晶电极和合成纳米粒子的电沉积利用了 Co 或 Fe 的存在,尤其是 Fe 表现出更高的活性:与纯氧化镍相比,塔菲尔斜率表明机理发生了显著变化,验证了在不同步骤激活 OER 的理论预测。含 25% Fe-Ni 的高性能合成纳米粒子的活性比 IrO2 提高了 4.6 倍,比 RuO2 提高了 34%,这表明非铂族金属催化剂的性能可以超过铂族金属。高分辨率透射电子显微镜进一步凸显了氧化铁-镍合成纳米粒子与商用催化剂相比的优势:与商用材料中典型的大纳米粒子相比,随机取向的小纳米粒子暴露出更大的边缘位点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


