Dual-Synergistic Nanomodulator Alleviates Exosomal PD-L1 Expression Enabling Exhausted Cytotoxic T Lymphocytes Rejuvenation for Potentiated iRFA-Treated Hepatocellular Carcinoma Immunotherapy
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The tumor immunosuppressive microenvironment (TME) induced by incomplete radiofrequency ablation (iRFA) in hepatocellular carcinoma (HCC) is a critical driver of tumor progression and metastasis. Herein, we proposed a therapeutic strategy aimed at remodeling the post-iRFA TME by targeting exosome biogenesis, secretion, and PD-L1 expression, thereby rejuvenating cytotoxic T lymphocyte function to mitigate the progression and metastasis of HCC. Leveraging the versatile properties of polydopamine nanomodulators, we have engineered a tailored delivery platform for GW4869 and amlodipine (AM), enabling precise and tumor-specific release of these therapeutic agents. Initially, GW4869, a neutral sphingomyelinase inhibitor, synergized with AM, an intracellular calcium modulator, to suppress exosome biogenesis and secretion. Subsequently, AM triggered the autophagic degradation of PD-L1. In vitro and in vivo experiments demonstrated that this synergistic approach significantly enhanced the robust activation and proliferation of various functional T-cell subsets following iRFA, particularly CD8+T cells, IFN-γ+ CD8+ cytotoxic T cells, natural killer cells, and innate lymphoid cells. Concurrently, it effectively reduced the infiltration of immunosuppressive cell types, including regulatory T cells and myeloid-derived suppressor cells. This favorable remodeling of the TME substantially inhibited the progression and metastasis of HCC post-iRFA. Collectively, our study presented a promising paradigm for enhancing HCC treatment efficacy by integrating radiofrequency ablation with advanced immune modulation strategies.
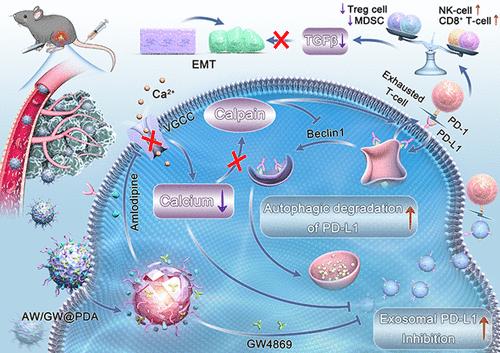
双重协同纳米调节剂可减轻外泌体 PD-L1 表达,使衰竭的细胞毒性 T 淋巴细胞恢复活力,促进 iRFA 治疗肝细胞癌的免疫疗法
肝细胞癌(HCC)不完全射频消融(iRFA)诱导的肿瘤免疫抑制微环境(TME)是肿瘤进展和转移的关键驱动因素。在此,我们提出了一种治疗策略,旨在通过靶向外泌体的生物生成、分泌和 PD-L1 表达来重塑 iRFA 后的 TME,从而恢复细胞毒性 T 淋巴细胞的功能,以缓解 HCC 的进展和转移。利用多巴胺纳米调节剂的多功能特性,我们为 GW4869 和氨氯地平(AM)设计了一个定制的递送平台,实现了这些治疗药物的精确和肿瘤特异性释放。起初,GW4869(一种中性鞘磷脂酶抑制剂)与AM(一种细胞内钙调节剂)协同抑制外泌体的生物生成和分泌。随后,AM 引发了 PD-L1 的自噬降解。体外和体内实验表明,这种协同方法能显著增强 iRFA 治疗后各种功能性 T 细胞亚群(尤其是 CD8+T 细胞、IFN-γ+ CD8+ 细胞毒性 T 细胞、自然杀伤细胞和先天性淋巴细胞)的活化和增殖。同时,它还有效减少了免疫抑制细胞类型的浸润,包括调节性 T 细胞和髓源性抑制细胞。TME的这种有利重塑大大抑制了iRFA术后HCC的进展和转移。总之,我们的研究为将射频消融与先进的免疫调节策略相结合以提高 HCC 治疗效果提供了一个前景广阔的范例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


