Photoinduced formal [4 + 2] cycloaddition of two electron-deficient olefins and its application to the synthesis of lucidumone
IF 14.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Electronically mismatched Diels−Alder reaction between two electron-deficient components is synthetically useful and yet underdeveloped under thermal conditions. Herein, a photoinduced formal [4 + 2] cycloaddition of enone with a variety of electron-deficient dienes is described. Key to the success of this stepwise methodology relies on a C − C bond cleavage/rearrangement of the cyclobutane based overbred intermediate via diversified mechanistic pathways. Based on this annulation method, total synthesis of lucidumone is achieved in nine steps.
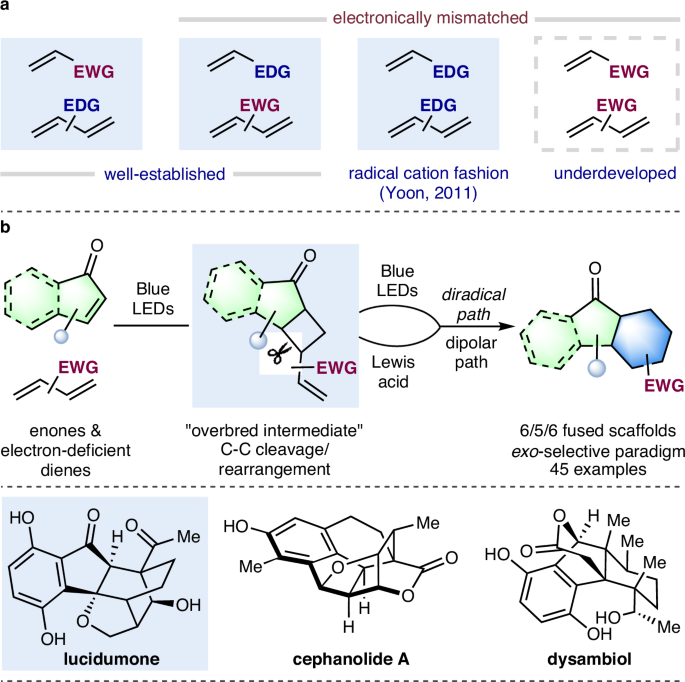
光诱导两种缺电子烯烃的正规[4 + 2]环加成及其在合成露西酮中的应用
两种缺电子成分之间的电子错配 Diels-Alder 反应在合成上非常有用,但在热条件下却没有得到充分发展。本文介绍了烯酮与多种缺电子二烯的光诱导正式[4 + 2]环加成反应。这种循序渐进的方法成功的关键在于通过多样化的机理途径对基于环丁烷的杂交中间体进行 C - C 键裂解/重排。基于这种环化方法,可通过九个步骤实现露地分散酮的全合成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Communications
Biological Science Disciplines-
CiteScore
24.90
自引率
2.40%
发文量
6928
审稿时长
3.7 months
期刊介绍:
Nature Communications, an open-access journal, publishes high-quality research spanning all areas of the natural sciences. Papers featured in the journal showcase significant advances relevant to specialists in each respective field. With a 2-year impact factor of 16.6 (2022) and a median time of 8 days from submission to the first editorial decision, Nature Communications is committed to rapid dissemination of research findings. As a multidisciplinary journal, it welcomes contributions from biological, health, physical, chemical, Earth, social, mathematical, applied, and engineering sciences, aiming to highlight important breakthroughs within each domain.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


