Genetic architecture of cerebrospinal fluid and brain metabolite levels and the genetic colocalization of metabolites with human traits
IF 31.7
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
Abstract
Brain metabolism perturbation can contribute to traits and diseases. We conducted a genome-wide association study for cerebrospinal fluid (CSF) and brain metabolite levels, identifying 205 independent associations (47.3% new signals, containing 11 new loci) for 139 CSF metabolites, and 32 independent associations (43.8% new signals, containing 4 new loci) for 31 brain metabolites. Of these, 96.9% (CSF) and 71.4% (brain) of the new signals belonged to previously analyzed metabolites in blood or urine. We integrated the metabolite quantitative trait loci (MQTLs) with 23 neurological, psychiatric and common human traits and diseases through colocalization to identify metabolites and biological processes implicated in these phenotypes. Combining CSF and brain, we identified 71 metabolite–trait associations, such as glycerophosphocholines with Alzheimer’s disease, O-sulfo-l-tyrosine with Parkinson’s disease, glycine, xanthine with waist-to-hip ratio and ergothioneine with inflammatory bowel disease. Our study expanded the knowledge of MQTLs in the central nervous system, providing insights into human traits. Genome-wide association study of cerebrospinal fluid and brain metabolites highlights the unique genetic architecture of metabolite levels and metabolite–trait associations with brain-related phenotypes.
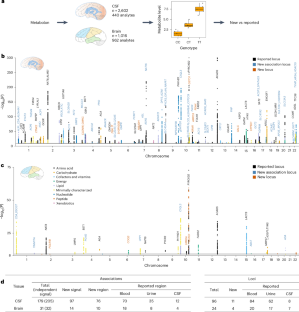

脑脊液和大脑代谢物水平的遗传结构以及代谢物与人类特征的遗传共定位
脑代谢紊乱可导致性状和疾病。我们对脑脊液(CSF)和脑代谢物水平进行了全基因组关联研究,为139种脑脊液代谢物确定了205个独立关联(47.3%的新信号,包含11个新位点),为31种脑代谢物确定了32个独立关联(43.8%的新信号,包含4个新位点)。其中,96.9%(脑脊液)和 71.4%(大脑)的新信号属于先前分析过的血液或尿液中的代谢物。我们通过共定位将代谢物定量性状位点(MQTLs)与 23 种神经、精神和人类常见性状和疾病结合起来,以确定与这些表型有关的代谢物和生物过程。结合脑脊液和大脑,我们发现了71种代谢物与性状的关联,如甘油磷酸胆碱与阿尔茨海默病的关联,O-硫代-l-酪氨酸与帕金森病的关联,甘氨酸、黄嘌呤与腰臀比的关联,麦角硫因与炎症性肠病的关联。我们的研究拓展了人们对中枢神经系统中MQTLs的认识,为人类的性状提供了洞察力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


