Structure of the yeast ceramide synthase
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Ceramides are essential lipids involved in forming complex sphingolipids and acting as signaling molecules. They result from the N-acylation of a sphingoid base and a CoA-activated fatty acid, a reaction catalyzed by the ceramide synthase (CerS) family of enzymes. Yet, the precise structural details and catalytic mechanisms of CerSs have remained elusive. Here we used cryo-electron microscopy single-particle analysis to unravel the structure of the yeast CerS complex in both an active and a fumonisin B1-inhibited state. Our results reveal the complex’s architecture as a dimer of Lip1 subunits bound to the catalytic subunits Lag1 and Lac1. Each catalytic subunit forms a hydrophobic crevice connecting the cytosolic site with the intermembrane space. The active site, located centrally in the tunnel, was resolved in a substrate preloaded state, representing one intermediate in ceramide synthesis. Our data provide evidence for competitive binding of fumonisin B1 to the acyl-CoA-binding tunnel. Using cryo-electron microscopy, Schäfer et al. solved the structure of the yeast ceramide synthase complex, consisting of Lip1, Lag1 and Lac1 subunits. They found that fumonisin B1 binds competitively at a key site, suggesting a mechanism for ceramide synthesis.

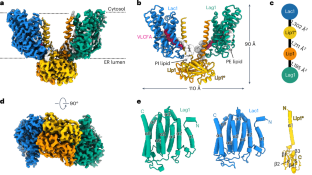
酵母神经酰胺合成酶的结构
神经酰胺是一种重要的脂质,可形成复杂的鞘脂并作为信号分子。神经酰胺是由鞘氨醇基和 CoA 激活的脂肪酸 N-酰化反应产生的,该反应由神经酰胺合成酶(CerS)家族的酶催化。然而,神经酰胺合成酶的精确结构细节和催化机理仍然难以捉摸。在这里,我们利用低温电子显微镜单粒子分析揭示了酵母 CerS 复合物在活性和伏马菌素 B1 抑制状态下的结构。我们的研究结果揭示了该复合体的结构,它是由 Lip1 亚基与催化亚基 Lag1 和 Lac1 结合而成的二聚体。每个催化亚基都形成了一个疏水缝隙,将细胞膜部位与膜间隙连接起来。位于隧道中心的活性位点在底物预载状态下被解析,代表了神经酰胺合成的一个中间过程。我们的数据为伏马菌素 B1 与酰基-CoA 结合隧道的竞争性结合提供了证据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


