Potent Amphiphilic Poly(Amino Acid) Nanoadjuvant Delivers Biomineralized Ovalbumin for Photothermal-Augmented Immunotherapy
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
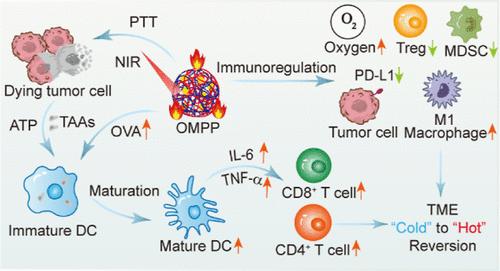
Cancer nanovaccines have emerged as an indispensable weapon for tumor treatment. However, insufficient immunogenicity and immunosuppression hamper the therapeutic effects of nanovaccines. Here, biodegradable nanovaccines (OMPP) composed of ovalbumin (OVA)-manganese oxide nanoparticles, amphiphilic poly(γ-glutamic acid) (γ-PGA), and ε-polylysine (PL) are constructed to realize enhanced cancer immunotherapy. Interestingly, amphiphilic γ-PGA and PL could serve as both carriers and immunoadjuvants to promote the cytosolic delivery of antigens and enhance the maturation of dendritic cells. Additionally, taking advantage of the photothermal property of OMPP, immunogenic cell death and in situ release of tumor-associated antigens can be triggered under near-infrared light irradiation for personalized tumor treatment. Moreover, OMPP nanovaccines can efficiently alleviate tumor hypoxia and downregulate programmed death-ligand 1 expression to reprogram the immunosuppressive tumor microenvironment. OMPP-mediated therapy has been shown to provoke robust immune responses to suppress B16-OVA melanoma and prevent postsurgical tumor recurrence. This work presents a facile strategy for the fabrication of nanovaccines by integrating carrier and adjuvant while exploring the inherent properties to promote antigen release and modulate immunosuppression, which demonstrates great potential for effective cancer immunotherapy.
为光热增强免疫疗法提供生物矿化卵清蛋白的强效两亲性聚(氨基酸)纳米佐剂
癌症纳米疫苗已成为治疗肿瘤不可或缺的武器。然而,免疫原性不足和免疫抑制阻碍了纳米疫苗的治疗效果。在此,研究人员构建了由卵清蛋白(OVA)-氧化锰纳米颗粒、两亲性聚(γ-谷氨酸)(γ-PGA)和ε-聚赖氨酸(PL)组成的生物可降解纳米疫苗(OMPP),以实现增强癌症免疫疗法。有趣的是,两亲的γ-PGA和PL既可以作为载体,也可以作为免疫佐剂,促进抗原的胞浆传递,并增强树突状细胞的成熟。此外,利用 OMPP 的光热特性,可在近红外线照射下触发免疫原性细胞死亡和肿瘤相关抗原的原位释放,从而实现个性化肿瘤治疗。此外,OMPP 纳米疫苗还能有效缓解肿瘤缺氧,下调程序性死亡配体 1 的表达,从而对免疫抑制性肿瘤微环境进行重编程。研究表明,OMPP 介导的疗法能激起强大的免疫反应,抑制 B16-OVA 黑色素瘤,防止手术后肿瘤复发。这项研究提出了一种简便的纳米疫苗制备策略,将载体和辅助剂结合在一起,同时探索其促进抗原释放和调节免疫抑制的固有特性,为有效的癌症免疫疗法展示了巨大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


