Adsorbability of a wide range of per- and polyfluoroalkyl substances on granular activated carbon, ion exchange resin, and surface modified clay
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
The increased detection of understudied per- and polyfluoroalkyl substances (PFAS) in environmental matrices has highlighted the need to evaluate the treatability of a wide-range of PFAS by sorption-based processes. This study investigated the efficacy of three commercial adsorbents (i.e., granular activated carbon (GAC), surface modified clay (SMC), and anionic exchange resin (AER)) for the removal of a wide range of cationic, zwitterionic, and anionic PFAS from an aqueous film forming foam (AFFF)-impacted groundwater employing rapid small-scale column tests (RSSCTs) coupled with high resolution mass spectrometry (HRMS) and suspect screening analysis (SQ). AER exhibited later breakthrough times for the majority of anionic and zwitterionic PFAS compared to SMC and GAC. However, both AER and SMC exhibited negligible removal of cationic PFAS presumably due to the reliance of these adsorbents on electrostatic interactions and the counteraction of hydrophobic forces caused by the repulsion between cationic PFAS and positively charged surfaces of AER and SMC. GAC, being a non-selective adsorbent, was largely unaffected by the ionic charge of the evaluated PFAS with molecular structure having a bigger impact on adsorbability. The detection of a variety of PFAS classes in the investigated AFFF-impacted groundwater enabled assessment of the relative impact of chemical structure on adsorptive removal of PFAS. Chain-length dependent adsorption was observed across all investigated anionic and zwitterionic PFAS classes. The PFAS structures possessing hydroxyl and/or methyl functional groups exhibited later breakthrough times compared to their homologues lacking these functional groups and cyclic/unsaturated structures were removed less efficiently compared to their linear/saturated homologues. In the case of perfluoroalkyl acid (PFAA)-derivative structures, hydrogen-substituted classes (i.e., H-PFAAs) were removed more efficiency than PFAAs while keto-substituted structures (i.e., K-PFSA) and pentahydrido-fluoroalkane sulfates (PeH-FAOS) exhibited lower adsorbability compared to PFAAs for all adsorbents. Oxa-PFAAs (O-PFSA; isomer class of PFA-OS) on the other hand demonstrated higher adsorbability compared to PFAAs in the case of AER-like adsorbents, while this trend was reversed for GAC.
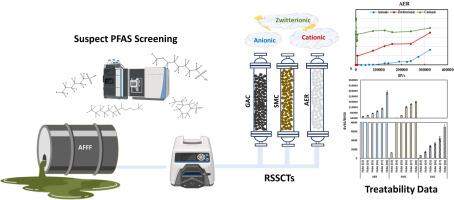
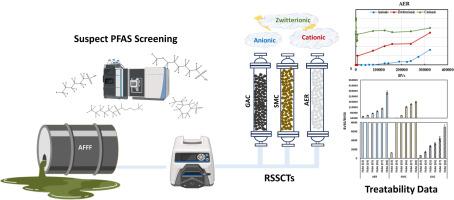
颗粒活性炭、离子交换树脂和表面改性粘土对多种全氟和多氟烷基物质的吸附性
环境基质中检测到的未充分研究的全氟和多氟烷基物质(PFAS)越来越多,这凸显了通过基于吸附的工艺来评估各种全氟和多氟烷基物质的可处理性的必要性。本研究采用快速小规模柱试验 (RSSCT)、高分辨率质谱分析 (HRMS) 和疑似筛选分析 (SQ),调查了三种商用吸附剂(即颗粒活性炭 (GAC)、表面改性粘土 (SMC) 和阴离子交换树脂 (AER))去除受水成膜泡沫 (AFFF) 影响的地下水中各种阳离子、齐聚阴离子和阴离子 PFAS 的功效。与 SMC 和 GAC 相比,AER 对大多数阴离子和齐聚物 PFAS 的突破时间较晚。不过,AER 和 SMC 对阳离子全氟辛烷磺酸的去除几乎可以忽略不计,这可能是由于这些吸附剂依赖于静电相互作用以及阳离子全氟辛烷磺酸与 AER 和 SMC 带正电的表面之间的排斥力所产生的疏水力的反作用。作为一种非选择性吸附剂,GAC 基本上不受所评估的全氟辛烷磺酸离子电荷的影响,而分子结构对吸附性的影响更大。在所调查的受 AFFF 影响的地下水中检测到了各种类别的 PFAS,这有助于评估化学结构对吸附去除 PFAS 的相对影响。在所有调查的阴离子和齐聚物全氟辛烷磺酸类别中,都观察到了链长依赖性吸附。与缺乏羟基和/或甲基官能团的同系物相比,具有羟基和/或甲基官能团的 PFAS 结构的突破时间较晚,与线性/饱和同系物相比,环状/不饱和结构的去除效率较低。就全氟烷基酸(PFAA)衍生物结构而言,氢取代类(即 H-PFAA)的去除效率高于全氟烷基酸,而酮取代结构(即 K-PFSA)和五水氟烷硫酸盐(PeH-FAOS)在所有吸附剂中的吸附性均低于全氟烷基酸。另一方面,在类似 AER 的吸附剂中,Oxa-PFAAs(O-PFSA;PFA-OS 的异构体类别)与 PFAAs 相比具有更高的吸附性,而对于 GAC 而言,这一趋势正好相反。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


