Self-catalytic enhancement of Cu-EDTA decomplexation and simultaneous Cu recovery via a dual-cathode electrochemical process
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Heavy metals that are readily chelated with coexisting organic ligands in industrial wastewaters impose threats to environment and human health but are also valuable metal resources. Traditional treatment methods generally require additional chemicals and generate secondary contaminants. Here, a reagent-free dual-cathode electrochemical system was proposed for the efficient destruction of Cu-organic complexes and synchronous cathodic recovery of Cu, whereby in situ production of H2O2 at carbon aerogel (CA) cathode was coupled with the reduction of Cu(II) to Cu(I) and finally to Cu(0) at Ti cathode. The intermediate Cu(II) complexes enabled the self-reinforced degradation owing to their higher activities toward •OH generation by activating H2O2 in contrast to initial Cu-ethylenediaminetetraacetic acid (Cu-EDTA). The enhanced production of Cu(I) by Ti cathode facilitated both •OH and Cu(III) formation, and the copper redox cycle was realized in the self-reinforced system, maintaining its sustainable catalytic activity. The energy cost of the dual-cathode system is 0.011 kWh/g for decomplexation and 0.057 kWh/g for Cu recovery, which is much lower than single Ti or CA cathode system. This established process provides a prospective approach for cost-effective destruction of chelating metal complexes and metal resources recovery from heavy metal wastewaters.
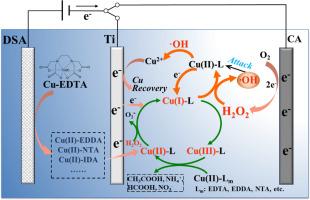
通过双阴极电化学过程自催化增强 Cu-EDTA 解络合并同时回收 Cu
工业废水中容易与共存有机配体螯合的重金属对环境和人类健康构成威胁,但同时也是宝贵的金属资源。传统的处理方法通常需要额外的化学品,并会产生二次污染。在此,我们提出了一种无试剂双阴极电化学系统,用于高效破坏铜有机络合物并同步阴极回收铜,即在碳气凝胶(CA)阴极原位产生 H2O2,同时在钛阴极将 Cu(II) 还原成 Cu(I) 并最终还原成 Cu(0)。与最初的铜-乙二胺四乙酸(Cu-EDTA)相比,中间的 Cu(II) 复合物通过激活 H2O2 生成-OH 的活性更高,因此能够实现自我强化降解。钛阴极产生的 Cu(I)的增强促进了 -OH 和 Cu(III)的形成,在自增强系统中实现了铜氧化还原循环,保持了其可持续的催化活性。双阴极系统的解络合能耗为 0.011 kWh/g,铜回收能耗为 0.057 kWh/g,远低于单 Ti 或 CA 阴极系统。这种成熟的工艺为经济有效地销毁螯合金属络合物和从重金属废水中回收金属资源提供了一种前景广阔的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


