Metabolite identification of emerging disinfection byproduct dibromo-benzoquinone in vivo and in vitro: Multi-strategy mass-spectrometry annotation and toxicity characterization
IF 10.3
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
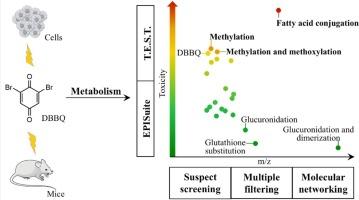
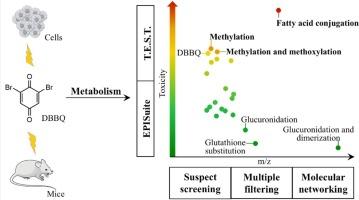
Halobenzoquinones (HBQs) are emerging disinfection byproducts (DBPs) of high toxicity and also are shared active toxic intermediates of multiple halogenated organic pollutants. Due to the strong oxidizing property and electrophilicity, HBQs exhibit extremely diverse metabolism pathways in organisms. The identification of toxic-decisive metabolites is pivotal, albeit challenging, for understanding the toxicity mechanisms of HBQs. We employed dibromo-benzoquinone (DBBQ) as a representative HBQ, and established a systematic analytical strategy using high-resolution mass spectrometry, which collectively coupled suspect screening (SS), mass defect filtering (MDF), product ion filtering (PIF), isotopic signature filtering (ISF), and molecular networking (MN). As a result, 20 biotransformation products of DBBQ were identified in vivo and in vitro, involving metabolism reactions such as hydroxylation, methylation, methoxylation, acetylation, sulfonation, glucuronidation, glutathionylation, dimerization, and conjugation with amino acids or fatty acids. Quantitative structure-activity relationship (QSAR) analysis and cytotoxicity experiments consistently demonstrated the significantly high toxicity of the fatty acid conjugate compared to the parent compound DBBQ and other metabolites, pinpointing the important role of the fatty acid conjugation in determining the metabolism and toxicity of HBQs. The research conducted a comprehensive evaluation of the metabolism of a typical HBQ with the combination of multiple analytical and toxicity characterization methods, therefore screen out the most important metabolism pathway of HBQs.
新出现的消毒副产物二溴苯醌在体内和体外的代谢物鉴定:多策略质谱注释和毒性表征
卤代苯醌(HBQs)是新出现的高毒性消毒副产物(DBPs),也是多种卤代有机污染物的共同活性毒性中间体。由于具有强氧化性和亲电性,HBQs 在生物体内的代谢途径极为多样。要了解 HBQs 的毒性机理,鉴定毒性决定性代谢物至关重要,尽管这具有挑战性。我们以二溴对苯醌(DBBQ)为代表性 HBQ,利用高分辨质谱建立了一套系统的分析策略,将疑似筛选(SS)、质量缺陷过滤(MDF)、产物离子过滤(PIF)、同位素特征过滤(ISF)和分子网络(MN)结合起来。结果发现了 20 种 DBBQ 在体内和体外的生物转化产物,涉及羟基化、甲基化、甲氧基化、乙酰化、磺化、葡萄糖醛酸化、谷胱甘肽化、二聚化以及与氨基酸或脂肪酸共轭等代谢反应。定量结构-活性关系(QSAR)分析和细胞毒性实验一致表明,与母体化合物 DBBQ 和其他代谢物相比,脂肪酸轭合物的毒性明显较高,从而明确了脂肪酸轭合物在决定 HBQs 代谢和毒性方面的重要作用。该研究结合多种分析和毒性表征方法,对典型 HBQ 的代谢进行了全面评估,从而筛选出 HBQ 最重要的代谢途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
文献相关原料
公司名称
产品信息
阿拉丁
Formic acid
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


