Assessing the discrepant role of anions in the transformation of reactive oxygen species in H2O2 and PDS system: A comparative kinetic analysis
IF 2.9
Q2 PUBLIC, ENVIRONMENTAL & OCCUPATIONAL HEALTH
引用次数: 0
Abstract
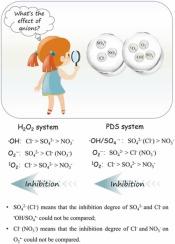
Clarifying reactive oxygen species (ROS) variation in the presence of co-existing anions is significant for understanding the catalytic effect of magnetite (Fe3O4)-induced advanced oxidation processes (AOPs) in natural environment, yet this remains controversial. Herein, we compare the specific impacts of NO3-, SO42-, and Cl- on ROS (•OH, SO4•-, O2•-, and 1O2) exposure concentration in H2O2 and peroxydisulfate (PDS) systems, as well as how these variations affect the catalytic efficiency by developing kinetic model. In both two systems, NO3- demonstrates no discernible effect on ROS, whereas SO42- inhibits the exposure of all ROS and thus micropollutants degradation. Through theoretical calculation, it is proposed that SO42- primarily exerts its influence through affecting the electronic structure over catalyst surface. Regarding Cl-, it affects ROS exposure mainly by reacting with ROS. It shows inhibitory effect on 1O2 in both systems, but its suppressive impact on •OH is markedly more pronounced in H2O2 system compared to PDS system, which may be related to its rapid reactivity with SO4•-. Besides, the chlorine radicals (mainly ClO•) generated through the reaction of Cl- may exert a selective influence on micropollutants degradation. This study can help to re-understand the influence behavior of co-existing anions during AOPs.
评估阴离子在 H2O2 和 PDS 系统中活性氧转化过程中的不同作用:动力学比较分析
弄清共存阴离子存在时活性氧(ROS)的变化对于理解自然环境中磁铁矿(Fe3O4)诱导的高级氧化过程(AOPs)的催化作用具有重要意义,但这一点仍然存在争议。在此,我们比较了在 H2O2 和过氧化二硫酸盐(PDS)体系中,NO3-、SO42- 和 Cl- 对 ROS(-OH、SO4-、O2-和 1O2)暴露浓度的具体影响,以及这些变化如何通过建立动力学模型影响催化效率。在这两个系统中,NO3- 对 ROS 没有明显的影响,而 SO42- 则会抑制所有 ROS 的暴露,从而抑制微污染物的降解。通过理论计算,可以认为 SO42- 主要通过影响催化剂表面的电子结构来施加影响。至于 SO42-,它主要通过与 ROS 发生反应来影响 ROS 暴露。在两种体系中,它对 1O2 都有抑制作用,但与 PDS 体系相比,它对 -OH 的抑制作用在 H2O2 体系中更为明显,这可能与它与 SO4- 的快速反应有关。此外,Cl-反应生成的氯自由基(主要是 ClO-)可能会对微污染物的降解产生选择性影响。这项研究有助于重新理解共存阴离子在 AOP 过程中的影响行为。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Chemical Health & Safety
PUBLIC, ENVIRONMENTAL & OCCUPATIONAL HEALTH-
CiteScore
3.10
自引率
20.00%
发文量
63
期刊介绍:
The Journal of Chemical Health and Safety focuses on news, information, and ideas relating to issues and advances in chemical health and safety. The Journal of Chemical Health and Safety covers up-to-the minute, in-depth views of safety issues ranging from OSHA and EPA regulations to the safe handling of hazardous waste, from the latest innovations in effective chemical hygiene practices to the courts'' most recent rulings on safety-related lawsuits. The Journal of Chemical Health and Safety presents real-world information that health, safety and environmental professionals and others responsible for the safety of their workplaces can put to use right away, identifying potential and developing safety concerns before they do real harm.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


