Optimizing Nonaqueous Sodium–Polysulfide Redox-Flow Batteries: The Role of Solvation Effects with Glyme Solvents
IF 19.3
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
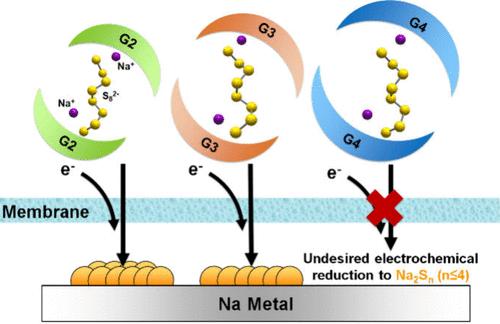
Nonaqueous redox-flow batteries (NARFBs) that use economical alkali metals and the corresponding metal polysulfides are highly attractive for grid-scale energy storage. Although sodium–sulfur systems have been recognized as promising candidates and have been the focus of many studies due to their high earth abundance and energy density, an understanding of the role of the solvation chemistry of commonly used glyme solvents is missing. Herein, we report a systematic investigation into the solvation effects of glyme-based Na-S electrolytes through comprehensive physiochemical experiments and Density Functional Theory (DFT) simulations. Our findings revealed, on one hand, that an optimal coordination strength between glymes and Na+ could maintain a relatively smooth Na+ diffusion. On the other hand, glyme solvents with extended chain lengths shift the reduction potential of S82– negatively to elevate the formation barrier of undesirable short-chain polysulfides (Sn2–, n ≤ 4) that have high membrane permeability. This solvation phenomenon not only mitigates capacity fading but also extends the operational longevity of the Na-S NARFBs. The results underscore the critical roles of balanced solvent–cation interactions and controlled redox potentials in improving the stability and efficiency of Na-S NARFB systems, marking a significant advancement in the development of sustainable energy storage solutions.
优化非水硫化钠-多硫化物氧化还原流电池:甘油溶剂溶解效应的作用
使用经济型碱金属和相应的金属多硫化物的非水氧化还原液流电池(NARFBs)对于电网规模的能量存储极具吸引力。尽管钠硫系统因其地球丰度高、能量密度大而被认为是有前途的候选物质,并成为许多研究的重点,但人们对常用乙二胺溶剂的溶解化学作用还缺乏了解。在此,我们报告了通过全面的物理化学实验和密度泛函理论(DFT)模拟对甘油基 Na-S 电解质的溶解效应进行的系统研究。我们的研究结果表明,一方面,乙二胺与 Na+ 之间的最佳配位强度可以保持 Na+ 相对平稳的扩散。另一方面,具有较长链长的甘油醚溶剂会使 S82- 的还原电位发生负向移动,从而提高具有较高膜渗透性的不良短链多硫化物(Sn2-,n ≤ 4)的形成障碍。这种溶解现象不仅缓解了容量衰减,还延长了 Na-S NARFB 的工作寿命。研究结果强调了平衡的溶剂-阳离子相互作用和受控的氧化还原电位在提高 Na-S NARFB 系统的稳定性和效率方面的关键作用,标志着在开发可持续能源存储解决方案方面取得了重大进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


