Unraveling the Charge Storage Mechanism of β-MnO2 in Aqueous Zinc Electrolytes
IF 19.3
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
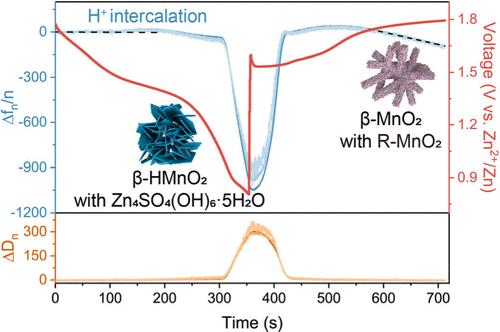
MnO2-based zinc-ion batteries have emerged as a promising candidate for next-generation energy storage systems. Despite extensive research on MnO2 electrodes, the charging mechanism in mildly acidic electrolytes remains debated. Most studies have focused on α-MnO2, and this study aims to shed light on the identity of the charge carrier in β-MnO2 and the role of the Mn2+ cations. By employing in situ EQCM-D measurements, along with ssNMR, XRD, TEM, and in situ pH monitoring, we demonstrated that the charging mechanism is primarily governed by proton de/intercalation. Compared to α-MnO2, with its larger 2 × 2 tunnels that accommodate hydronium ions, the β-phase has smaller 1 × 1 tunnels, permitting only the insertion of bare protons. During cycling, we observed the formation of new phases on β-MnO2 originating from the repetitive electrodeposition/dissolution of Mn2+. In addition, these phases can reversibly host hydronium ions, resulting in a mixed charging mechanism that involves the insertion of both H3O+ and H+.
揭示锌水电解质中 β-MnO2 的电荷存储机制
基于二氧化锰的锌离子电池已成为下一代储能系统的理想候选材料。尽管对二氧化锰电极进行了广泛研究,但在弱酸性电解质中的充电机制仍存在争议。大多数研究都集中在 α-MnO2 上,本研究旨在揭示 β-MnO2 中电荷载体的身份以及 Mn2+ 阳离子的作用。通过原位 EQCM-D 测量、ssNMR、XRD、TEM 和原位 pH 值监测,我们证明了充电机制主要受质子脱出/插层的支配。α-MnO2 的 2 × 2 通道较大,可容纳氢离子,相比之下,β 相的 1 × 1 通道较小,只允许裸质子插入。在循环过程中,我们观察到由于 Mn2+ 的重复电沉积/溶解,β-MnO2 上形成了新的相。此外,这些相还能可逆地容纳氢离子,从而形成一种混合充电机制,包括 H3O+ 和 H+ 的插入。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


