Polyurethane foam acidolysis with carboxylic acids: acid structure dictates N-containing product distribution and kinetics†
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
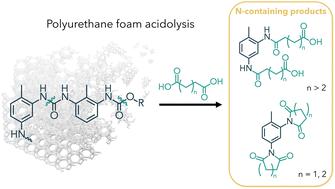
Obtaining circularity will be essential in managing plastic waste and moving towards sustainable materials. Chemical recycling offers a pathway to obtain valuable molecules from plastic waste, closing the loop on what is currently a linear economy. Here, we report on the chemical recycling of polyurethane foam (PUF) via acidolysis with dicarboxylic acids (DCAs) to release value-added molecules. While previous work has explored recovery of the recycled polyol (repolyol), we focus in this report on elucidating the product distribution and kinetics of the nitrogen-containing products from the acidolysis reaction. Using Nuclear Magnetic Resonance (NMR) spectroscopy and Ultra-High Pressure Liquid Chromatography – Mass Spectroscopy (UPLC-MS), we demonstrate how acid loading and structure influence product distribution of acidolysis. The use of excess acid can eliminate oligomeric content and aromatic amines from the product mixture. With DCAs composed of 2 or 3 carbons between the carboxylic acid groups, we observe the formation of imide products during acidolysis, which has only very recently been reported in the literature. Furthermore, the kinetics of imide formation were investigated and modeled for glutaric acid (GA) and succinic acid (SA), which form 6- and 5-membered cyclic imides, respectively. Di-imide formation with SA proceeds without accumulation of intermediates and is an order of magnitude faster than imide formation with GA, for which an amide-imide intermediate is detected and the rate of reaction is sensitive to steric hindrance. This report offers fundamental insights into the N-containing products formed during acidolysis, which will aid scale-up of closed-loop chemical recycling processes.
聚氨酯泡沫与羧酸的酸解:酸结构决定含 N 产物的分布和动力学
实现循环对管理塑料废弃物和向可持续材料发展至关重要。化学回收为从塑料废弃物中获取有价值的分子提供了一条途径,使目前的线性经济实现了闭环。在此,我们报告了聚氨酯泡沫(PUF)的化学回收,通过二羧酸(DCA)的酸解作用释放出高附加值分子。之前的研究已经探讨了回收多元醇(repolyol)的回收问题,而本报告的重点是阐明酸解反应中含氮产物的分布和动力学。利用核磁共振(NMR)光谱和超高压液相色谱-质谱(UPLC-MS)技术,我们展示了酸负荷和结构如何影响酸解产物的分布。使用过量的酸可以消除产品混合物中的低聚物含量和芳香胺。对于在羧酸基团之间含有 2 或 3 个碳原子的 DCAs,我们观察到在酸解过程中会形成亚胺产物,而这种现象最近才在文献中有所报道。此外,我们还对戊二酸(GA)和琥珀酸(SA)分别形成 6 元和 5 元环状亚胺的亚胺形成动力学进行了研究和建模。与 SA 形成二酰亚胺的过程中没有中间产物的积累,比与 GA 形成酰亚胺的过程快一个数量级,后者可检测到酰胺酰亚胺中间产物,而且反应速度对立体阻碍很敏感。本报告提供了有关酸解过程中形成的含 N 产物的基本见解,这将有助于扩大闭环化学循环过程的规模。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


