Structural and Photophysical Differences in Crystalline Trigonal Planar Copper Iodide Complexes with 1,2-Bis(methylpyridin-2-yl)disilane Ligands
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
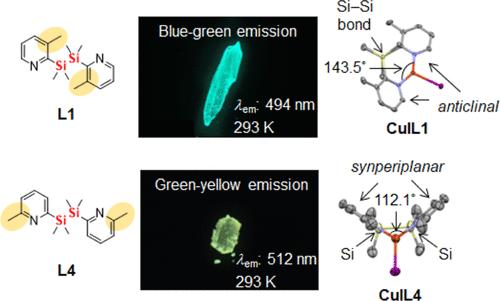
We synthesized trigonal planar Cu(I) iodide complexes with 1,2-bis(methylpyridin-2-yl)disilane ligands L1–L4 and investigated how the substitution position of the methyl group on the pyridine ring in σ–π conjugation affects their structure and physical properties. The structures were characterized by NMR, elemental analysis, and single-crystal X-ray diffraction. In the crystalline state, the methylpyridyl groups of CuIL1–CuIL3 were coordinated with Cu(I) in an anticlinal conformation relative to the Si–Si σ bond, whereas those of CuIL4 were coordinated with Cu(I) in a synperiplanar conformation relative to the Si–Si σ bond. The conformational difference in the crystalline state was influenced by the N−Cu−N bite angle and the emission wavelength. CuIL1–CuIL3 exhibited blue-green emission (λem: 476–494 nm), and CuIL4 exhibited green-yellow emission (λem: 512 nm) with high emission quantum yields (Φ: 0.59–0.86) in the crystalline state at 293 K. These Cu(I) complexes exhibited thermally activated delayed fluorescence from the S1 state at 293 K and phosphorescence from the T1 state at 77 K in the crystalline state. The optical properties in the crystalline state were discussed by DFT and TD-DFT calculations. These complexes also displayed aggregation-induced emission in THF–water solution (fw > 80%), although they did not show emission in dehydrated THF.
含 1,2-双(甲基吡啶-2-基)二硅烷配体的晶体三方平面碘化铜配合物的结构和光物理差异
我们合成了具有 1,2-双(甲基吡啶-2-基)二硅烷配体 L1-L4 的三方平面碘化铜(I)配合物,并研究了σ-π共轭吡啶环上甲基的取代位置如何影响其结构和物理性质。核磁共振、元素分析和单晶 X 射线衍射对这些化合物的结构进行了表征。在结晶状态下,CuIL1-CuIL3 的甲基吡啶基与 Cu(I)配位,相对于硅-硅 σ 键呈反侧构象,而 CuIL4 的甲基吡啶基与 Cu(I)配位,相对于硅-硅 σ 键呈同侧构象。结晶态中的构象差异受 N-Cu-N 咬合角和发射波长的影响。CuIL1-CuIL3 发出蓝绿色荧光(λem:476-494 nm),CuIL4 发出绿黄色荧光(λem:512 nm),在 293 K 的结晶态下具有较高的发射量子产率(Φ:0.59-0.86)。这些 Cu(I)配合物在结晶态下表现出 S1 态在 293 K 下的热激活延迟荧光和 T1 态在 77 K 下的磷光。通过 DFT 和 TD-DFT 计算讨论了结晶态的光学特性。这些复合物在四氢呋喃-水溶液(fw > 80%)中也显示出聚集诱导的发射,但在脱水四氢呋喃中没有显示出发射。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


