Zincophilic zwitterionic hydrogel electrolyte towards dendrite-free zinc ion hybrid supercapacitors with anti-self-discharge ability
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
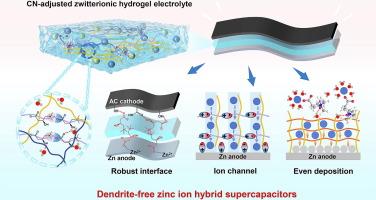
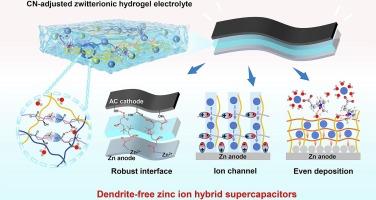
Hydrogel-based zinc ion hybrid supercapacitors (ZHSCs) with high flexibility and appealing energy/power densities have attracted increasing attention in energy storage fields. However, the Zn dendrite growth, water-related side reactions, and fast self-charge issues lead to severe performance degeneration of hydrogel-based ZHSCs. Herein, a zwitterion L-carnitine (CN) was integrated into hydrogel electrolyte to construct a dendrite-free and anti-corrosive hydrogel-based ZHSC with a slow self-discharge rate. CN can enter the Zn2+ inner solvation sheath and isolate active water molecules from Zn anodes to induce homogeneous Zn2+ deposition and inhibit undesired side reactions. The CN ionic complexes serving as ion transport channels enable the Zn2+ to move along the migration channels, endowing the hydrogel electrolytes with a high Zn2+ transference number of 0.847. Besides, CN with zwitterionic groups forms electrostatic interactions with cations and anions, which prevents the diffusion of moveable ions from the electrode surfaces to hydrogel electrolytes, thus effectively prolonging the self-discharge time of ZHSCs. Accordingly, the hydrogel-based ZHSCs exhibit dendrite-free and corrosive-free behaviors on Zn electrodes after long-time operation, presenting a capacitance retention of 75 % after 2000 charge and discharge cycles at 1 A/g. The hydrogel-based ZHSC also display a long self-discharge time of 3h, which is more than 9 times that of the one without CN. This work provides guidance on the design of dendrite-free and anti-self-discharge hydrogel-based zinc ion energy storage devices.
亲锌齐聚物水凝胶电解质用于制造具有抗自放电能力的无树枝状杂化锌离子超级电容器
水凝胶基锌离子混合超级电容器(ZHSCs)具有高柔性和诱人的能量/功率密度,在储能领域受到越来越多的关注。然而,锌枝晶的生长、与水有关的副反应以及快速自充电等问题导致水凝胶基锌离子混合超级电容器的性能严重下降。在此,研究人员在水凝胶电解质中加入了滋养剂左旋肉碱(CN),从而构建了一种无枝晶、抗腐蚀、自放电速度慢的水凝胶基 ZHSC。CN 可以进入 Zn2+ 内溶解鞘,并从 Zn 阳极隔离出活性水分子,从而诱导均匀的 Zn2+ 沉积,抑制不希望发生的副反应。作为离子传输通道的 CN 离子络合物可使 Zn2+ 沿迁移通道移动,从而使水凝胶电解质的 Zn2+ 迁移数高达 0.847。此外,带有齐聚物基团的 CN 与阳离子和阴离子形成静电相互作用,阻止了可移动离子从电极表面向水凝胶电解质的扩散,从而有效延长了 ZHSCs 的自放电时间。因此,水凝胶基 ZHSCs 在 Zn 电极上长时间工作后表现出无树枝状突起和无腐蚀性,在 1 A/g 条件下充放电 2000 次后电容保持率达到 75%。水凝胶基 ZHSC 的自放电时间也长达 3 小时,是不含 CN 的 ZHSC 的 9 倍多。这项研究为设计无树枝状突起、抗自放电的水凝胶基锌离子储能器件提供了指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


