Machine learning-based rational design for efficient discovery of allatostatin analogs as promising lead candidates for novel IGRs
Abstract
BACKGROUND
Insect neuropeptide allatostatins (ASTs) play a vital role in regulating insect growth, development, and reproduction, making them potential candidates for new insect growth regulators (IGRs). However, the practical use of natural ASTs in pest management is constrained by their long sequences and high production costs, thus the development of AST analogs with shorter sequences and reduced cost is essential. Traditional methods for designing AST analogs are often time-consuming and resource-intensive. This study aims to employ new computational methodologies to understand the structure–activity relationship and efficiently discover potent AST analogs.
RESULTS
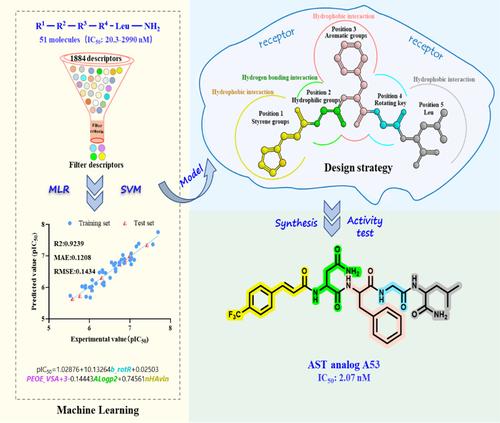
Two machine learning models, utilizing multiple linear regression and support vector machine, were constructed to reveal the key structural factors that influence the juvenile hormone-inhibiting activity of AST analogs. These models suggested that a potent AST analog should contain styrene, hydrophilic, and aromatic groups, and rotatable bonds at positions 1, 2, 3, and 4, respectively. Six analogs (A52-A57) were designed and synthesized, and they exhibited potent juvenile hormone-inhibiting activity (IC50 < 16 nM). Notably, analog A53 showed the best activity (IC50 = 2.07 nM), surpassing that of most natural Dippu-ASTs, making it a potential lead candidate for IGRs.
CONCLUSION
These models promote the efficient design, screening, and prioritization of new or untested AST analogs. The study clarifies how a machine learning-based strategy facilitates the development of AST analogs as novel IGR lead candidates, offering a useful reference for pest management. © 2024 Society of Chemical Industry.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


