Engineered manganese-BODIPY coordinated nanoadjuvants for enhanced NIR-II photo-metalloimmunotherapy
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
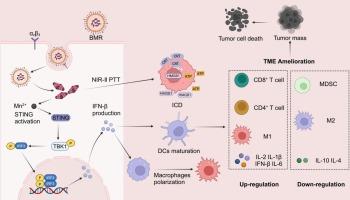
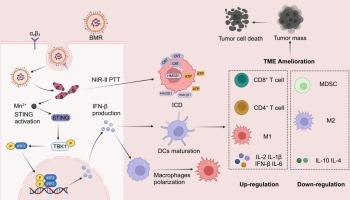
Immunotherapy, a pivotal and promising approach for tumor treatment, has demonstrated prominent clinical efficacy. However, its effectiveness is often impeded by insufficient antitumor immune responses attributed to the immunosuppressive tumor microenvironment (TME). The combination of immune activation through the stimulator of interferon genes (STING) pathway and phototherapy holds great potential for surmounting this challenge in advanced tumor immunotherapy. Herein, a novel manganese-boosted NIR-II photo-metalloimmunotherapy is proposed to synergistically enhance antitumour efficacy by fabricating Mn2+-BODIPY-based coordinated photo-immune nanoadjuvants (BMR), modified with tumor-targeted peptide cRGD. The obtained BMR could effectively deliver Mn2+ to tumor sites, and immunogenic cell death (ICD) was evoked by localized photothermal ablation of tumors using NIR-II laser irradiation. Simultaneously, pH-responsive release of Mn2+ would trigger the activation of STING pathway to promote the production of type I interferons (I-IFNs), significantly facilitating the maturation of dendritic cells (DCs) and polarization of macrophages to M1 phenotypes. Furthermore, by synergistically initiating systematic and robust antitumour immune responses, the BMR-mediated NIR-II photo-metalloimmunotherapy achieved remarkable therapeutic efficacy against both primary and lung metastasis of B16F10 tumors. Overall, in light of the versatile functionalities and synthetic flexibility of coordinated nanoadjuvants, formulated with photofunctional ligands and diverse metal ions, this work provides new insights into the design of metal coordination nanomedicine for effective antitumor photo-metalloimmunotherapy.
用于增强近红外-II 光金属免疫疗法的工程锰-BODIPY 配位纳米佐剂
免疫疗法是治疗肿瘤的一种关键且前景广阔的方法,已显示出显著的临床疗效。然而,免疫抑制性肿瘤微环境(TME)导致的抗肿瘤免疫反应不足往往会阻碍免疫疗法的效果。通过干扰素基因刺激器(STING)途径激活免疫反应与光疗相结合,有望克服晚期肿瘤免疫疗法面临的这一挑战。本文提出了一种新型的锰促进近红外-II光金属免疫疗法,通过制备基于Mn2+-BODIPY的协同光免疫纳米佐剂(BMR),并用肿瘤靶向肽cRGD修饰,协同提高抗肿瘤疗效。所获得的 BMR 能有效地将 Mn2+ 递送到肿瘤部位,并利用近红外-II 激光照射肿瘤局部光热消融诱发免疫性细胞死亡(ICD)。同时,Mn2+的pH响应性释放会触发STING通路的激活,促进I型干扰素(I-IFNs)的产生,从而显著促进树突状细胞(DCs)的成熟和巨噬细胞向M1表型的极化。此外,BMR 介导的近红外-II 光金属免疫疗法通过协同启动系统而强大的抗肿瘤免疫反应,对 B16F10 肿瘤的原发和肺转移取得了显著疗效。总之,鉴于配位纳米佐剂具有多功能性和合成灵活性,并由光功能配体和多种金属离子配制而成,这项工作为设计金属配位纳米药物以实现有效的抗肿瘤光-金属免疫疗法提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
文献相关原料
公司名称
产品信息
阿拉丁
Pyrrolidine
阿拉丁
acetic acid
阿拉丁
N, N-dimethylformamide (DMF)
阿拉丁
manganese chloride (MnCl2)
阿拉丁
triethylamine
阿拉丁
1,3-diphenylisobenzofuran (DPBF)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


