Cation-induced topical disordered niobium nickel oxide for robust hydrogen storage in magnesium hydride
IF 15.8
1区 材料科学
Q1 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
Abstract
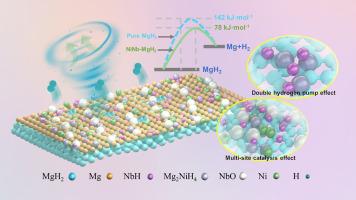
Catalytic doping is one of the economic and efficient strategies to optimize the operating temperature and kinetic behavior of magnesium hydride (MgH2). Herein, efficient regulation of electronic and structural rearrangements in niobium-rich nickel oxides was achieved through precise compositional design and niobium cation functionalized doping, thereby greatly enhancing its intrinsic catalytic activity in hydrogen storage systems. As the niobium concentration increased, the Ni-Nb catalysts transformed into a mixed state of multi-phase nanoparticles (composed of nickel and niobium-rich nickel oxides) with smaller particle size and uniform distribution, thus exposing more nucleation sites and diffusion channels at the MgH2/Mg interface. In addition, the additional generation of active Ni-Nb-O mixed phase induced numerous highly topical disordered and distorted crystalline, promoting the transfer and reorganization of H atoms. As a result, a stable and continuous multi-phase/component synergistic catalytic microenvironment could be constructed, exerting remarkable enhancement on MgH2's hydrogen storage performance. After comparative tests, Ni0.7Nb0.3-doped MgH2 presented the optimal low-temperature kinetics with a dehydrogenation activation energy of 78.8 kJ·mol−1. The onset dehydrogenation temperature of MgH2+10 wt% Ni0.7Nb0.3 was reduced to 198 °C and 6.18 wt% H2 could be released at 250 °C within 10 min. In addition, the dehydrogenated MgH2 NiNb composites absorbed 4.87 wt% H2 in 10 min at 125 °C and a capacity retention rate was maintained at 6.18 wt% even after 50 reaction cycles. In a word, our work supplies fresh insights for designing novel defective-state multiphase catalysts for hydrogen storage and other energy related field.
NiNb composites absorbed 4.87 wt% H2 in 10 min at 125 °C and a capacity retention rate was maintained at 6.18 wt% even after 50 reaction cycles. In a word, our work supplies fresh insights for designing novel defective-state multiphase catalysts for hydrogen storage and other energy related field.
阳离子诱导的局部无序氧化铌镍在氢化镁中实现稳健储氢
催化掺杂是优化氢化镁(MgH2)操作温度和动力学行为的经济而有效的策略之一。在这里,通过精确的成分设计和铌阳离子功能化掺杂,实现了对富铌镍氧化物中电子和结构重排的有效调节,从而大大提高了其在储氢系统中的内在催化活性。随着铌浓度的增加,镍-铌催化剂转变为粒径更小、分布更均匀的多相纳米颗粒(由镍和富铌镍氧化物组成)的混合状态,从而在 MgH2/Mg 界面暴露出更多的成核点和扩散通道。此外,活性镍-铌-氧化物混合相的额外生成诱导了大量高度拓扑无序和扭曲的晶体,促进了 H 原子的转移和重组。因此,可以构建一个稳定、连续的多相/组分协同催化微环境,显著提高 MgH2 的储氢性能。经过对比试验,掺杂 Ni0.7Nb0.3 的 MgH2 具有最佳的低温动力学性能,其脱氢活化能为 78.8 kJ-mol-1。MgH2+10 wt% Ni0.7Nb0.3的起始脱氢温度降低到198 °C,在250 °C的温度下,10分钟内可释放出6.18 wt%的H2。此外,脱氢的 MgH2NiNb 复合材料在 125 ℃ 下 10 分钟内吸收了 4.87 wt% 的 H2,即使经过 50 个反应循环,容量保持率仍为 6.18 wt%。总之,我们的工作为设计新型缺陷态多相催化剂用于储氢和其他能源相关领域提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Magnesium and Alloys
Engineering-Mechanics of Materials
CiteScore
20.20
自引率
14.80%
发文量
52
审稿时长
59 days
期刊介绍:
The Journal of Magnesium and Alloys serves as a global platform for both theoretical and experimental studies in magnesium science and engineering. It welcomes submissions investigating various scientific and engineering factors impacting the metallurgy, processing, microstructure, properties, and applications of magnesium and alloys. The journal covers all aspects of magnesium and alloy research, including raw materials, alloy casting, extrusion and deformation, corrosion and surface treatment, joining and machining, simulation and modeling, microstructure evolution and mechanical properties, new alloy development, magnesium-based composites, bio-materials and energy materials, applications, and recycling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


