Discovery of novel thiosemicarbazone-acridine targeting butyrylcholinesterase with antioxidant, metal complexing and neuroprotector abilities as potential treatment of Alzheimer's disease: In vitro, in vivo, and in silico studies
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
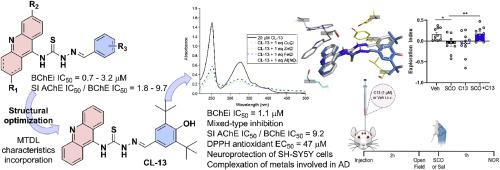
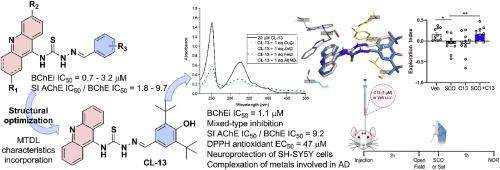
Inhibition of cholinesterases, combined with antioxidant activity, metal-chelating capacity, and neuroprotection, is recognized as an effective multitarget therapy for the treatment of Alzheimer's disease (AD). Based on our in-house thiosemicarbazone-acridine compounds, this study recognized these derivatives as possible multi-target-directed ligand (MTDL). Initial screening against cholinesterases identified CL-01, which exhibited a promising IC50 value of 0.71 μM against butyrylcholinesterase (BChE). Twelve new derivatives were designed based on CL-01 aiming to retain the BChE inhibitory activity while incorporating a MTDL profile, including antioxidant properties and metal-complexing abilities. Among the new derivatives, CL-13 maintained a good BChE inhibition (IC50 = 1.15 μM) with improved selective index against acetylcholinesterase (SI = 9.2). The acridine nucleus was important for the activity, as its saturated tetrahydroacridine analogue (TA-01) showed a decrease in cholinesterases inhibition potencies and altered the mode of inhibition, revealing for the first time distinct functional roles for the two nuclei. Moreover, CL-13 emerged as a promising lead compound, demonstrating interesting antioxidant activity (DPPH EC50 = 47.01 μM), chelating capacity of biometals involved in Aβ aggregation and/or oxidative stress, and a lack of neurotoxicity at 50 μM in SH-SY5Y cells. It also exhibited neuroprotective effects in an in vitro oxidative stress model induced by H2O2. Finally, in vivo experiments confirmed that CL-13 effectively reversed scopolamine-induced cognitive impairment, without affecting locomotor activity in the mice.
发现具有抗氧化、金属络合和神经保护能力的新型硫代氨基羰基吖啶丁酰胆碱酯酶,作为阿尔茨海默病的潜在治疗药物:体外、体内和硅学研究。
抑制胆碱酯酶与抗氧化活性、金属螯合能力和神经保护相结合,被认为是治疗阿尔茨海默病(AD)的有效多靶点疗法。基于我们内部的硫代氨基脲-吖啶化合物,本研究将这些衍生物视为可能的多靶点配体(MTDL)。对胆碱酯酶的初步筛选确定了 CL-01,它对丁酰胆碱酯酶(BChE)的 IC50 值为 0.71 μM,前景看好。在 CL-01 的基础上设计了 12 种新的衍生物,旨在保留 BChE 抑制活性的同时融入 MTDL 特性,包括抗氧化特性和金属络合能力。在这些新衍生物中,CL-13 保持了良好的 BChE 抑制作用(IC50 = 1.15 μM),并提高了对乙酰胆碱酯酶的选择性指数(SI = 9.2)。吖啶核对其活性非常重要,因为其饱和四氢吖啶类似物(TA-01)显示出胆碱酯酶抑制效力下降,并改变了抑制模式,首次揭示了两个核的不同功能作用。此外,CL-13 还是一种很有前途的先导化合物,它具有有趣的抗氧化活性(DPPH EC50 = 47.01 μM),对参与 Aβ 聚集和/或氧化应激的生物金属具有螯合能力,并且在 SH-SY5Y 细胞中 50 μM 的浓度下没有神经毒性。在 H2O2 诱导的体外氧化应激模型中,它还表现出神经保护作用。最后,体内实验证实,CL-13 能有效逆转东莨菪碱诱导的小鼠认知障碍,且不影响小鼠的运动活动。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


