Dysfunction of exhausted T cells is enforced by MCT11-mediated lactate metabolism
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
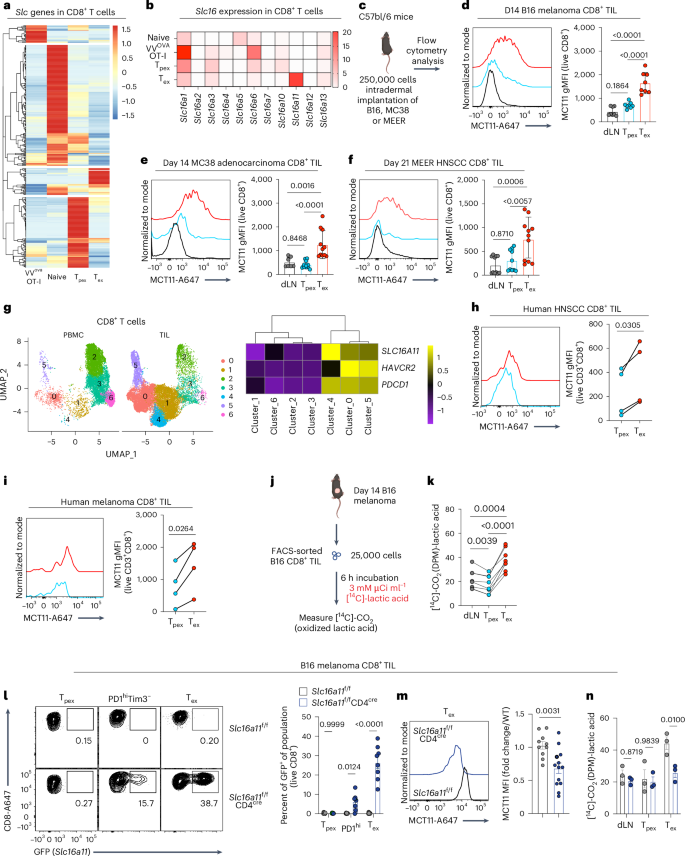
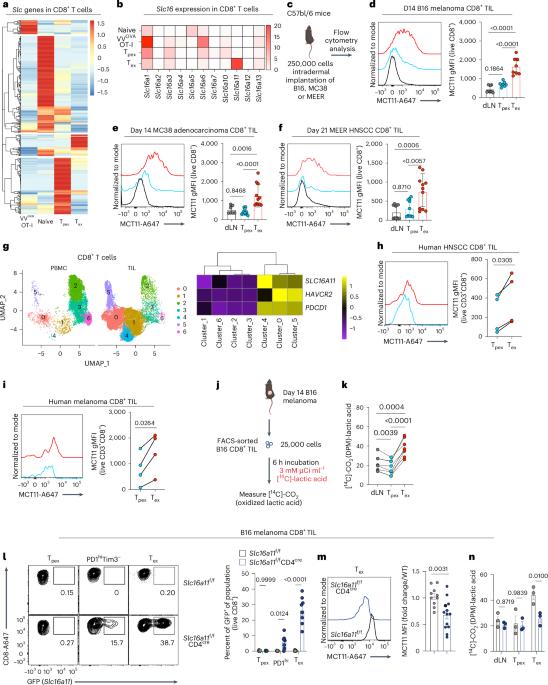
CD8+ T cells are critical mediators of antitumor immunity but differentiate into a dysfunctional state, known as T cell exhaustion, after persistent T cell receptor stimulation in the tumor microenvironment (TME). Exhausted T (Tex) cells are characterized by upregulation of coinhibitory molecules and reduced polyfunctionality. T cells in the TME experience an immunosuppressive metabolic environment via reduced levels of nutrients and oxygen and a buildup of lactic acid. Here we show that terminally Tex cells uniquely upregulate Slc16a11, which encodes monocarboxylate transporter 11 (MCT11). Conditional deletion of MCT11 in T cells reduced lactic acid uptake by Tex cells and improved their effector function. Targeting MCT11 with an antibody reduced lactate uptake specifically in Tex cells, which, when used therapeutically in tumor-bearing mice, resulted in reduced tumor growth. These data support a model in which Tex cells upregulate MCT11, rendering them sensitive to lactic acid present at high levels in the TME. Here the authors show that high expression of MCT11 is key to the dysfunctionality associated with exhausted CD8+ T cells in tumors. By targeting MCT11, uptake of lactic acid, which is abundant in the tumor, is reduced, resulting in improved effector functions and tumor immunity.
MCT11 介导的乳酸代谢强化了衰竭 T 细胞的功能障碍
CD8+ T 细胞是抗肿瘤免疫的关键介质,但在肿瘤微环境(TME)中持续受到 T 细胞受体刺激后,会分化成一种功能失调状态,即 T 细胞衰竭。衰竭T细胞(Tex)的特点是共抑制分子上调和多功能性降低。肿瘤微环境中的 T 细胞因营养和氧气水平降低以及乳酸堆积而处于免疫抑制的代谢环境中。在这里,我们发现终末Tex细胞独特地上调编码单羧酸转运体11(MCT11)的Slc16a11。在 T 细胞中条件性缺失 MCT11 会减少 Tex 细胞对乳酸的吸收,并改善其效应功能。用抗体靶向 MCT11 可减少 Tex 细胞对乳酸的特异性摄取,在对肿瘤小鼠进行治疗时,可减少肿瘤的生长。这些数据支持一种模型,即 Tex 细胞上调 MCT11,使其对 TME 中高浓度的乳酸敏感。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


